Figure 7
Download original image
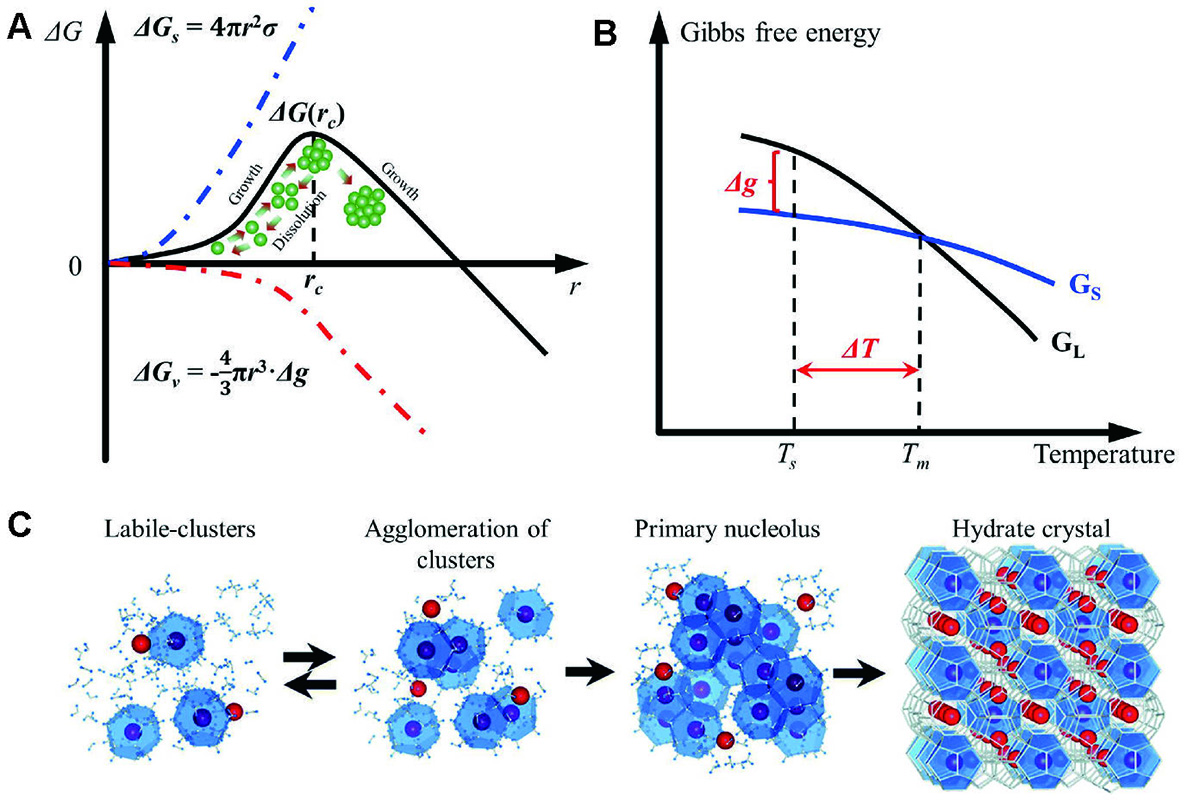
Variation of Gibbs free energy for a transition from the liquid state to solid state. (A) Relationship between the nucleation radius and Gibbs free energy. (B) Relationship between the temperature and Gibbs free energy. (C) Labile-cluster model of hydrate nucleation [37]. Copyright © 2020, Royal Society of Chemistry. Note: ΔG denotes the Gibbs free energy; ΔG(r c) denotes the Gibbs free energy corresponding to the critical radius; Δg and ΔT denotes the difference in free energy per unit volume and supercooling degree of PCMs, respectively.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

