| Issue |
Natl Sci Open
Volume 3, Number 3, 2024
|
|
|---|---|---|
| Article Number | 20230017 | |
| Number of page(s) | 40 | |
| Section | Earth and Environmental Sciences | |
| DOI | https://doi.org/10.1360/nso/20230017 | |
| Published online | 09 November 2023 | |
REVIEW
Potential and prospects in molecular orbital level micro-electric field for low energy consumption water purification
Institute of Environmental Research at Greater Bay Area, Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou 510006, China
* Corresponding author (email: huchun@gzhu.edu.cn)
Received:
16
March
2023
Revised:
2
June
2023
Accepted:
4
July
2023
Conventional water purification technologies struggle to simultaneously address purification efficiency and energy consumption. Molecular orbital level surface micro-electric field (MEF)-driven water purification is an original and innovative concept conceived and developed by our group in recent years. The core idea involves creating electron-rich and electron-poor micro-areas on the nanomaterial surface, which drive pollutants or H2O molecules to provide electrons in the electron-poor micro-areas while other environmental factors (such as H2O2 and O2) obtain electrons in the electron-rich micro-areas. This process effectively utilizes the internal energy contained within wastewater and emerging contaminants (ECs). Centered on this core, this review systematically examines the discovery, construction, and characteristics of MEF and MEF-like systems and summarizes their application directions. The challenges, bottlenecks, and future development directions of MEF technology are also analyzed and discussed. Reviews of MEFs can facilitate the development of low-consumption, high-efficiency water purification technologies.
Key words: molecular orbital / micro-electric field / low energy consumption / water purification / pollutant utilization
© The Author(s) 2023. Published by Science Press and EDP Sciences.
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Providing safe water is an increasingly severe challenge as new pollutants continuously emerge in the era of rapid global industrialization [1]. Water pollution is exacerbated by emerging contaminants (ECs), making it more complex, difficult, and expensive to treat [2]. Currently, over 650 million people worldwide lack access to safe drinking water [3]. Alarmingly, every two minutes, a child under the age of five succumbs to preventable diarrheal diseases caused by poor water quality [4]. ECs, the primary culprits that threaten the ecological environment and cause these serious health events, have been frequently detected at levels in natural water environments, effluent from sewage treatment plants, and even drinking water sources [2]. This fact underscores the importance and urgency of disruptive innovation in water purification technology [5], particularly for the removal of refractory ECs from water.
Advanced oxidation technologies (AOTs) that employ highly oxidizing species, such as hydroxyl radicals (•OH) and superoxide radicals (O2•–), as attacking factors have been found to possess the ability to eliminate organic pollutants that are difficult to biodegrade [6–8]. In particular, the rapid development of nanotechnology has brought in a new era for heterogeneous AOTs [9]. By regulating the size, morphology, and surface structure of nanomaterials, AOTs are endowed with water purification properties integrating adsorption, coagulation, catalysis, oxidation, and confined reactions [3], enabling rapid elimination of various pollutants in developed AOT systems [7]. These systems include Fenton/Fenton-like systems [10–13], persulfate activation systems [14–16], catalytic ozonation systems [17–19], photocatalytic systems [20–22], and electrocatalytic systems [23,24]. These processes primarily rely on the generation of free radicals to attack and degrade organic pollutants. However, high energy consumption is a significant drawback of AOTs due to the inherent limitations of these technologies, including the demand for large amounts of oxidants and reductants as well as the necessity for driving forces such as light, electricity, and ultrasonic energy [25]. Given the increasingly prominent constraints of global resources and the environment, the development of next-generation water treatment technology for EC elimination, focusing on low energy consumption and high efficiency, is essential.
Numerous studies have shown that current AOTs are primarily surface reactions, highlighting the key role of material surface characteristics. A series of recent studies have emphasized that the construction of different defects on the material surface based on molecular orbital polarization is conducive to the formation of confined electron-rich/poor micro-areas, which results in the formation of surface micro-electric field (MEF) and manifests as surface electrostatic forces [6,8,26–29]. As depicted in Figure 1, the polarization distribution of electrons is achieved by constructing electron-rich/poor micro-areas on the catalyst surface. This drives electron-rich substances, such as pollutants and H2O, to provide electrons and energy in the electron-poor micro-areas via electrostatic adsorption and complexation, thereby decomposing the pollutants. Simultaneously, other environmental factors, such as H2O2 and O2, are activated by obtaining electrons and energy in the electron-rich micro-areas. Different environmental factor-mediated activation processes have led to the evolution of various water purification technologies, including dual-reaction-center (DRC) Fenton-like processes, persulfate activation processes, photocatalysis, and water self-purification processes. The MEF-based process is found to be beneficial for utilizing internal energy, including pollutants, dissolved oxygen, salt ions in water, and even water molecules, through electron flow, energy flow, and mass flow. This provides a new direction for the development of low-energy-consumption water purification technologies.
 |
Figure 1 Common interfacial reaction mechanism of the MEF-based technologies. |
Centered on this core, this review systematically examines the discovery, construction, types, and main characteristics of surface MEFs and similar configurations associated with MEFs (MEF-like configurations). It thoroughly explains and summarizes their application research directions in the field of water purification, particularly for EC elimination. These applications include DRC-based Fenton-like processes for water purification, persulfate activation processes for water purification, photocatalysis for water purification, and water self-purification through internal energy utilization. The challenges, bottlenecks, and future development directions of MEF and MEF-like technologies are also analyzed and discussed.
Construction and properties of surface MEF on catalyst
Origin of surface MEF
In most developed materials, inevitable defects are always generated on the surface. However, these microstructures do not reflect the characteristics and performance of MEFs and cannot drive the migration and transformation of pollutant electrons. The key to forming directional MEFs with the function of pollutant energy utilization is regulating oriented electron flows at the molecular orbital level, which does not require additional energy. Generally, molecular orbital theory holds that electrons in a molecule no longer belong to a specific atom but move throughout the entire range of the molecule. Molecular orbitals are linear combinations of atomic orbitals of atoms in a molecule, which can form bonding molecular orbitals (such as σ and π orbitals) and antibonding molecular orbitals (such as σ* and π* orbitals) (Figure 2). Similarly, in solid materials, the outer electrons of internal atoms are also strongly influenced by neighboring atoms. Based on the different electronegativity and electrochemical characteristics of the atoms, the overlap of various atomic orbitals leads to the formation of strong interactions at the molecular orbital level, providing the possibility of non-equilibrium and the polarization distribution of electrons in a confined area on the material surface.
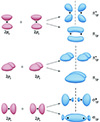 |
Figure 2 Molecular orbitals obtained from linear combination of atomic orbitals (LCAO) in molecules. |
The most common methods to generate electron-oriented migration at the molecular orbital level include lattice substitution by elements with varying electronegativity, surface complexation based on cation-π interactions, and interfacial coupling based on metal-organic polymerization. Specifically, cation-π interactions are among the most important intermolecular binding forces occurring between cations and electron-rich π orbitals [30]. The interaction between cations and delocalized π orbitals perpendicular to the plane of the aromatic ring often results in particularly strong cation-π binding, which surpasses the strength of hydrogen bonds and even charge-charge interactions in aqueous solutions [31]. The orbital interactions of cation-π typically involve electron transfer of the type π → cation (σ donation) and cation → π* (π back-donation) [30]. This electron transfer form can be easily regulated by reasonable structural modifications. It has been demonstrated that interfacial confinement can fundamentally alter the energetics of cation-π-mediated assembly [31]. Regulating the polarity enhancement of electrons and energy on the material surface through cation-π interactions is promising.
Based on the aforementioned theoretical foundation, we summarize the concept of surface MEF for the first time. Surface MEF is induced by the non-equilibrium distribution of electrons on the catalyst surface through the interaction of multiple molecular orbitals and is manifested as a surface electrostatic force. In recent years, various types of surface MEFs have been successfully constructed using lattice substitution [6,27,32], surface coordination complexation [8,26,33,34], interfacial coupling and polymerization [35], and molecular doping [36] to trigger the uneven distribution of molecular orbital electrons on material surfaces. Compared with conventional Fe-C materials that use their potential difference (~1.2 V) to promote Fe corrosion to degrade pollutants [37,38], the generated surface MEF on the catalyst consistently maintains a steady state both at room temperature without additional energy input and during pollutant degradation. This demonstrates a very promising application prospect for environmental remediation, as well as the energy and chemical industries.
Design and characterizations of surface MEF
Surface MEF construction through lattice substitution
The design concept of MEF involves modulating the catalyst structure so that electrons are nonuniformly distributed, forming electron density polarization centers. For metal oxide matrices, the solubility differences among various doping elements induce distinct deposition behaviors on the metal oxide matrix, resulting in their successful substitution into the matrix lattice during preparation [39]. The different electronegativities of doped elements create a synergistic effect, which alters the electronic orbit and lowers the Fermi energy level of the matrix, leading to charge redistribution primarily occurring around the doped elements [40,41]. Based on the electronegativity differences of various elements, the initial configuration of surface MEF, composed of electron-rich Cu areas and electron-poor Ti/Al areas (Figure 3 [42]), was constructed on d-TiCuAl-SiO2 Ns through lattice substitution [27].
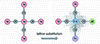 |
Figure 3 Schematic digram of electron cloud density distribution on d-TiCuAl-SiO2 Ns. Adapted with permission from ref. [42], Copyright©2017, Progress in Chemistry. |
On this basis, MEFs were successfully constructed by doping Mo, Fe, Co and Cu into the ZnS and Al2O3 lattice, respectively [6,25,43–46], and theoretically verified using density functional theory (DFT) calculations. For instance, lattice-doping Co into ZnO (OV-CoZnO MPs) induced a significant number of oxygen vacancies (OVs) at the site adjacent to Co atoms, as confirmed by X-ray photoelectron spectroscopy (XPS), Raman, and photoluminescence spectrum (PLS) analyses [38]. According to electron paramagnetic resonance (EPR) results, OV-CoZnO MPs exhibited a stronger EPR signal of unpaired electrons at g = 2.097 than ZnO, confirming that lattice doping with Co enabled more electrons to gather at oxygen vacancies through the formed -Zn-OV-Co-O- or -Co-OV-Zn-O- structural units, creating electron-rich OV centers and electron-deficient Co3+ sites. It was also found that OVs with more electrons formed in [Bi2O2S]2+ layers after S doping in BiOBr [47]. The same phenomenon regarding OVs was observed in the Fenton-like catalyst BiOCl [48]. These results indicated that OVs caused by lattice substitution functioned as electronic carriers and could serve as electronegative centers of the catalyst, deepening the understanding of constructing surface MEFs over catalysts. Furthermore, it was reported that substitutional doping with heteroatoms into the carbon skeleton could generate a unique electron distribution [49,50]. The doped heteroatom could introduce electronic states near the carbon Fermi energy level in the carbon framework, thus inducing local high spin density on the basal plane [51]. Based on this theory, numerous studies have been dedicated to the construction of surface MEFs for metal-free catalysts [52,53].
Surface MEF construction through surface complexation
In the aforementioned studies, the electronegativity difference of each element was utilized to modulate the non-uniform electron distribution, contributing to the formation of MEF on the catalyst surface. However, the intensity of the constructed MEF based on the electronegativity difference is quite limited, making the enhancement of the electronic structure and the construction of a stronger MEF challenging. Cation-π (M+-π) interactions, resulting from surface complexation, are electrostatic attractions between cations and electron-rich π orbitals. These interactions are stronger than hydrogen bonds and may even surpass charge-charge interactions [31,54–56]. The synergistic σ donation from the π system into an empty orbital of a metal and π back-donation of electron density from the occupied metal d orbitals into the π orbitals of the π system can significantly affect the structural and energetic parameters of the cation-π configuration [57]. In such a system, achieving directed and ordered migration of electrons in a specific manner may be key to overcoming the current bottleneck and building an efficient and stable surface MEF through cation-π interactions. Moreover, the electron density of the aromatic ring can be altered by replacing functional groups, resulting in an asymmetric potential of π binding to the ring and modifying the bond strength of the cation-π interactions [30]. Consequently, the intensity of the surface MEF can be progressively strengthened by constructing single, double, or even multiple cation-π electrostatic forces.
An electron distribution polarity-enhanced catalyst (OH-CCN/CuCo-Al2O3) was developed for the first time, consisting of Cu and Co co-substituted γ-Al2O3 combined with a carbon nitride (g-C3N4) substrate via a surface complexation method [8]. XPS, Fourier transform infrared spectroscopy (FTIR), and extended X-ray absorption fine structure (EXAFS) analyses confirmed the complexation of Cu species and the phenolic hydroxyl group of g-C3N4. EPR results revealed that the strong signal observed in OH-CCN/CuCo-Al2O3 was attributable to the increased electron density around Cu due to σ donation (π → Cu) of the Cu(II)-π interactions, which implied the formation of electron-rich Cu areas in OH-CCN/CuCo-Al2O3. This conjecture was confirmed by DFT calculations. Cu species in the C–O–Cu cross-linker had the maximum valence-electron density of ~ 14.19 e Å−3, while the lowest valence-electron density was observed around the C atoms of g-C3N4. Inspired by this discovery, a more efficient MEF was constructed on a CuAlO2 support with surface growth of g-C3N4 (CN-Cu(II)-CuAlO2) by increasing the connection between Cu and g-C3N4. According to DFT calculations, the maximum valence-electron density (~ 4.0 e Å−3) was found around Cu, and the lowest valence-electron density (~ 0.2 e Å−3) was found around C atoms [26]. Similarly, stable MEFs on transition metals (e.g., Fe, Cu, Co, V, Mo and Zn) doped with g-C3N4, reduced graphene oxide (rGO), polyimide (PI), carbon, boron nitride (BN), and chicken manures were successfully constructed using a similar method [58–68].
Surface MEF construction through interfacial coupling
Cation-π interactions can be enhanced by interfacial coupling, particularly the polar coupling process caused by nano zero-valent metal species, which often induces the utilization of multiple environmental factors. Interfacial coupling is considered another critical way to enhance the polarity of surface MEFs. Cao et al. [35] developed the RSC-CNOP using a surface reduction modification method with a Cu-PI precursor. The generated Cu(0) and Cu(I) on the catalyst surface triggered the anomalous bidirectional electron transfer to O atoms, forming electron-rich O centers with 8-fold higher free electrons than conventional catalysts. Based on this, a nanoscale Cu(0) combined with Cu(II)-doped rGO (nZVCu-Cu(II)-rGO) was prepared using a modified annealing reduction method [69]. EPR analysis demonstrated that charge transfer from the π-electronic reservoir of the underlying graphene sheet to the introduced Cu (σ donation) was enhanced with increasing Cu species, resulting in a large number of electrons accumulating around Cu species. The increased electron polarization of the surface structure led to the formation of an electron-rich Cu center and an electron-deficient C center with valence-electron densities of approximately 4.0 and 0.81 e Å−3, respectively, as verified by DFT calculations. Moreover, based on interface coupling theory, the construction of surface MEFs in Cu0@CuOx-encapsulated nitrogen-doped graphitic carbon (Cu0@CuOx-NC), nZVI/FeOx/FeNy-anchored NC composites (nZVI/FeN5.5O7.2/C), and MOF-coated Cu(0) (ZVC@CMOF) catalysts has also been investigated [10,70,71]. Recently, a Fe-based composite catalyst (Fe0-FeyCz/Fex-GZIF-8-rGO) was developed through interfacial three-phase coupling [34]. DFT calculations demonstrated that the successful coupling among the three phases triggered more electrons transferred from GZIF-8/rGO to the generated Fe0, forming an extremely strong MEF on the Fe0-FeyCz/Fex-GZIF-8-rGO surface, with the surface potential energy as high as 663.96 kJ mol−1. Therefore, the MEF on Fe0-FeyCz/Fex-GZIF-8-rGO could reduce the chemical bond energy of surface-adsorbed molecules without additional energy assistance, which might be very promising for low-energy water purification.
Interaction forms of MEF and different substances
In addition to some metal oxide MEF-containing catalysts, most MEF-containing catalysts developed thus far are carbon-based materials. During the reaction, it was found that water molecules could complex with the electron-deficient area of MEF to form an H-bonding molecular orbital interaction and delocalize electrons to the electron-rich area, resulting in oxidation into •OH [58]. In such a situation, the hydrogen peroxide (H2O2) added during the Fenton-like process could not directly interact with the electron-deficient area but instead bonded to the OH of the complexed H2O through a hydrogen bond (H–O–O•••H–O), thus hindering the oxidation of H2O2 [36]. However, in the presence of pollutants, the adsorbed pollutants could further replace the adsorbed water molecules and complex with the electron-deficient areas through π-π and H-bonding to form a surface-confined non-equilibrium electron orbital [36]. Thus, the adsorbed pollutants and their intermediates tended to delocalize electrons to the electron-rich areas through these strong electrostatic forces, enhancing catalyst stability [72,73]. Due to the occupation of the adsorbed pollutants at electron-deficient areas, H2O2 tended to adsorb on electron-rich areas through electrostatic force and was then directly and efficiently reduced to •OH, significantly improving H2O2 utilization. Han et al. [58] and Lu et al. [25] also found that dissolved oxygen (DO) confined in electron-rich areas could trap the delocalized electrons from H2O/pollutants and then undergo a surface reduction process to form O2•−/H2O2/•OH for pollutant degradation. Moreover, Wang et al. [34] reported that an extremely strong surface MEF could significantly reduce the chemical bonding energy of pollutants with strong molecular orbital interactions with electron-deficient areas. Specifically, through the electron delocalization effect of the surface MEF, the pollutants were first cleaved and then underwent hydrolytic hydroxylation and mineralization processes at room temperature, with O2 acting as an electron-acceptor in electron-deficient areas. Based on the unique surface characteristics of MEF, MEF-containing catalysts exhibit high degradation efficiency for various pollutants [26,52,53]. According to the aforementioned discoveries, the conversion pattern of surface-adsorbed substances depends on the intensity and structural characteristics of the MEF. Therefore, the directional regulation of the MEF structure allows it to play different roles in different systems. In the following sections, we will detail the role and application of surface MEF in various systems.
MEF driving DRC Fenton-like process for water purification
The Fenton reaction, one of the most powerful AOTs, has been widely used to eliminate ECs from water by generating •OH through the reaction of H2O2 and Fe2+ or other reducing transition metals [6]. The development of heterogeneous Fenton/Fenton-like catalysts has significantly overcome the weaknesses of homogeneous reactions, such as a narrow working pH range, iron sludge accumulation, and difficulty in recycling [10]. However, the basic Fenton reaction principle remains unchanged, relying on the redox of H2O2 at single sites. The existence of a rate-limiting step is inevitable, often leading to low utilization efficiency of H2O2 and poor activity, especially under neutral conditions. The DRC Fenton-like process based on surface MEFs offers hope for solving these problems through different types, which can drive various environmental factors to participate in electron acquisition and supply in electron-rich/poor micro-areas, thereby reducing H2O2 consumption [65].
Lattice substitution-type MEF driving DRC process
Based on the lattice substitution method, in 2016, one kind of Fenton-like catalyst consisting of dandelion-like copper-aluminum co-doped silica nanospheres (DCAS Ns) with a polarization distribution of surface electrons was first developed through an enhanced hydrothermal method (Figure 4A) [9]. The substitution of Cu and Al for Si resulted in a non-uniform distribution of electrons and the formation of different electrical regions, significantly increasing the number of reaction sites (Figure 4B). The successful formation of Al-O-Cu bonding bridges was confirmed by the variations in Cu and Al bonding energy before and after doping in XPS spectra (Figure 4C), as well as the variations in bond distance and coordination number of the Cu K-edge in EXAFS (Figure 4D). This catalyst exhibited exceptionally high activity and stability for the degradation and mineralization of various refractory pollutants under neutral conditions. The reaction rate was 5.2 to 13.4 times higher than that of the conventional Fenton catalysts (Figure 4E), accompanied by a high H2O2 utilization efficiency of 86.7% (Figure 4F). The efficient utilization of pollutant electrons by DCAS Ns was demonstrated by the 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO) spin-trapping EPR technique, which greatly promoted the generation of •OH and prevented the formation of HO2•/O2•− (Figure 4G).
 |
Figure 4 (A) TEM image and FESEM of DCAS Ns. (B) Schematic illustration of pollutants or H2O2 in contact with the catalyst with the active component in the bulk, the multiparous catalyst and DCAS Ns. (C) XPS spectra for Cu 2p3/2 for DCS Ns and Cu 2p3/2 for DCAS Ns. (D) EXAFS curve fitting of DCS Ns and DCAS Ns (solid line-experimental signals, dotted line-fitted curves). (E) BPA degradation and TOC removal in different suspensions with H2O2. (F) H2O2 decomposition and the utilization efficiency of H2O2 during the degradation of BPA in the DCAS Ns suspension. (G) BMPO spin trapping ESR spectra recorded at ambient temperature for various DCAS Ns samples with H2O2 in aqueous dispersion for BMPO–•OH and in methanol dispersion for BMPO–HO2•/O2•−: (1) fresh DCAS Ns; (2) DCAS Ns adsorbed BPA; and (3) DCAS Ns used for 15 min in the Fenton reaction with BPA. Adapted with permission from ref. [9], Copyright©2016, The Royal Society of Chemistry. |
In this approach, Ti, the third metal, was introduced to co-doped SiO2 nanospheres with Cu and Al (d-TiCuAl-SiO2 Ns), which further enhanced the polarization distribution of surface electrons. The Fenton-like reaction mechanism and H2O2 utilization efficiency were proposed and elucidated [27]. The successful formation of the electron-rich Cu region and the electron-poor Al/Ti region (M-O-M type MEF, where M represents metal species) was clearly demonstrated by EXAFS (Figure 5A), EPR (Figure 5B), XPS (Figure 5C), FTIR and cyclic voltammetry (Figure 5D) measurements. By utilizing the properties of different micro-regions of DRCs, H2O2 predominantly gained electrons in the electron-rich Cu region to generate •OH, and pollutants were oxidized in the electron-poor Ti/Al center for donating electrons. This resulted in nearly all the energy of H2O2 being applied to the degradation of pollutants. Separation of oxidation and reduction reactions led to reaction rates ~10-fold higher than traditional Fenton catalysts (Figure 5E) and 60% to 90% H2O2 utilization efficiency for the entire process (Figure 5F). This work suggested that the construction of an M-O-M type of MEF by lattice substitution could avoid the inefficient decomposition of H2O2 and significantly improve the Fenton-like catalytic activity.
 |
Figure 5 (A) Fourier transforms of k3-weighted EXAFS oscillations obtained at the Cu K-edge of the various samples. (B) Solid EPR spectra of various samples. (C) Cu 2p3/2 XPS spectra for d-TiCuAl-SiO2 Ns, d-Cu-SiO2, Ns d-CuAl-SiO2 Ns and Ti 2p XPS spectra for d-TiCuAl-SiO2 Ns. The inset shows the LMM X-ray induced Auger parameter for the sample. (D) CV of various electrodes prepared from the corresponding samples. (E) Fenton catalytic degradation of BPA in various suspensions at initial pH 7. (F) The utilization efficiency of H2O2 in the d-Cu-SiO2 Ns, d-CuAl-SiO2 Ns and d-TiCuAl-SiO2 Ns suspensions. The inset shows the corresponding decomposition of H2O2. Adapted with permission from ref. [27], Copyright©2016, The Royal Society of Chemistry. |
The construction of surface DRCs by utilizing metal electronegativity differences to modulate electron distribution has been demonstrated to be viable. Additionally, substituted M-O-M type MEFs (Zn, Cu, Co, Bi, etc.) with different metal elements were developed to further investigate the optimal synergistic patterns between MEF and H2O2. Notably, in the study of a Co-doped ZnO (OV-CoZnO MPs) Fenton-like system [6], it was found that lattice-doping Co into ZnO wurtzite can lead to the generation of OVs with unpaired electrons (electron-rich OV regions), while an electron-poor region forms around Co3+ (Figures 6A–6C). In this particular surface MEF, the efficient transformation and degradation of pollutants through multiple pathways over a wide pH range (4.5–9.5) were achieved through the synergistic interaction between the poor/rich microregions on this MEF, with reaction rates up to ~17 times that of the non-MEF catalyst (Figure 6D). Moreover, under molecular orbital action, pollutants were adsorbed on the electron-poor Co3+ region as electron donors to the system and were oxidized, thus achieving rapid electron cycling within this DRC system through the Co–OV bonding bridge (Figure 6E).
 |
Figure 6 (A) Fourier transforms spectra of Zn K-edge EXAFS for OV-CoZnO MPs and ZnO. (B) Raman spectra of the OV-CoZnO MPs and ZnO samples. (C) EPR spectra of the OV-CoZnO MPs, OV(L)-CoZnO MPs and ZnO solid samples. (D) RhB degradation curves in various suspensions with H2O2 (the inset shows the pseudo-first-order kinetic rate plots of ZnO and OV-CoZnO MPs Fenton-like systems). (E) Optimized adsorption/reaction model for H2O2 and typical hydroxylated pollutant fragment (phenol) on the surface of OV-CoZnO MPs through DFT calculations. DFT total energy (EDFT) and adsorption energy (ΔE) for H2O2 and phenol at different sites on the surface of OV-CoZnO MPs. Adapted with permission from ref. [6], Copyright©2020, American Chemical Society. |
The aforementioned work indicated that OVs formed by metal doping indeed diversified the types of surface MEFs and greatly enhanced the molecular orbital polarization between the catalyst surface and the H2O2/pollutants. Additionally, the formation of OVs was also observed in surface MEFs developed with non-metal-doped metal oxides. In the case of I-doped BiO (f-BiOI MSs) [32], electron-rich oxygen regions and electron-poor OV regions were successfully constructed. The (101) and (110) crystal planes in this catalyst were greatly exposed, providing numerous sites for the reaction. The rapid removal of refractory bisphenol A (BPA) by the Fenton-like reaction could be achieved within 3 min, with a reaction rate ~190-fold higher than that of the traditional Fenton catalyst, Fe2O3. The electron-rich oxygen region could effectively reduce H2O2 to •OH, while the electron-poor OVs captured electrons from the adsorbed pollutants and transferred them to the electron-rich region. With the synergistic effect of H2O2, this surface DRC was able to achieve rapid degradation and mineralization of pollutants in a wide pH range.
We have expanded the construction of MEFs beyond oxide substrates to sulfides (M-S-M type MEF) and obtained promising results. In a study of Fe-doped ZnS quantum dots (Fe-ZnS QDs) [43], non-equilibrium surface DRCs with electron polarization distribution were successfully constructed by replacing a portion of Zn with Fe using an enhanced in situ synthesis method (Figure S1A). Due to the construction of the DRCs on the Fe-ZnS QD surface, trace H2O2 (less than 1 mmol L−1) activation overcame the energy barrier for pollutant electron transfer (Figures S1B and S1C). Rapid pollutant removal was then achieved by reactive oxygen species (ROS) activated from DO utilizing pollutant electrons and energy, reaching 97.2% in just 15 s. The effect of pollutant structure on MEF performance was also explored (Figure S1D). Generally, pollutants with phenolic hydroxyl groups and carboxyl groups were more likely to interact with MEF, with electrons being utilized by cation-π interactions and other mechanisms. As a result, the removal efficiency for BPA by this MEF was significantly higher than that of SMZ. Experimental and theoretical studies demonstrated that constructing DRCs on the surface of Fe-ZnS quantum dots greatly strengthened the orbital interactions between each molecule and the catalyst surface, allowing pollutant electrons to be efficiently utilized for H2O2 reduction while DO was activated by obtaining energy and electrons (Figure S1E). The construction of this specific MEF enabled the rapid removal of pollutants with the assistance of only trace amounts of H2O2, significantly reducing energy consumption in the Fenton reaction.
Cation-π-type MEF driving pollutant utilization
The lattice substitution of metal elements to construct MEFs has proven to be an effective method in Fenton/Fenton-like reactions. However, due to the extreme values of elemental electronegativity, there is a limit to the construction. Cation-π (M+-π) interactions were introduced to improve this limitation through surface complexation. In 2017, the Cation-π-type Fenton-like catalyst OH-CCN/CuCo-Al2O3 was successfully developed by complexing CuCo-Al2O3 with g-C3N4 through the C–O–Cu bonding bridge (Figures 7A and 7B) [8]. EPR spectroscopy (Figure 7C) and DFT calculations (Figure 7D) verified the generation of an enhanced electron-rich region around Cu due to Cu-π interactions and electronegativity differences among Cu, Co, and Al through the construction of C–O–Cu bonding bridges. Driven by the cation-π electrostatic force, the amount of •OH generated under near-neutral conditions was twice the amount of H2O2 involved in the reaction. Additionally, H2O served as an electron donor and was oxidized into •OH at the N atom of OH-CCN, resulting in rapid pollutant removal with ~90% H2O2 utilization and a high turnover frequency (TOF) of the •OH yield (1.30 s−1), which was more than ~85 times that of the traditional homogeneous Fenton reaction (Figures 7E and 7F). This work suggested that utilizing cationic-π interactions is an effective way to enhance surface electron polarization distribution, enabling MEFs to utilize pollutant energy as well as environmental factors (H2O, O2, and more) under molecular orbital interactions.
 |
Figure 7 (A) Fourier transforms of k3-weighted EXAFS oscillations obtained at the Cu K-edge. (B) XPS spectra in Cu 2p3/2 for CuCo-Al2O3 and OH-CCN/CuCo-Al2O3. (C) EPR spectra of all of the fresh solid samples. (D) Degradation of BPA in various suspensions with H2O2 and TOC removal during the degradation of BPA in various suspensions. (E) The structure and two-dimensional valence-electron density color-filled maps of carbon-doped g-C3N4 growing on the surface of Cu-doped g-Al2O3 through the connection of C-O-Cu: the valence-electron density map in the Cu-O1-C1 plane, O1-C1-C2 plane and O1-C1-N1 plane. (F) BMPO spin-trapping EPR spectra for HO2•/O2•− and •OH in various methanol dispersions without H2O2. Adapted with permission from ref. [8], Copyright©2017, The Royal Society of Chemistry. |
Subsequently, a more efficient surface complexation cation-π-type MEF (CN-Cu(II)-CuAlO2) was developed by growing g-C3N4 on a CuAlO2 substrate via a C–O–Cu bonding bridge (Figures 8A–8C) [26]. Due to the surface growth of this linker on the MEF surface, a Cu-rich electron region was formed with greatly reduced surface OVs, resulting in high activity and efficiency for the degradation of refractory pollutants (Figure 8D) while maintaining high H2O2 utilization (Figure 8E). During the Fenton-like reactions, the electron-rich Cu region was responsible for the efficient reduction of H2O2 to •OH, and the electron-poor C region captured electrons from H2O2 or pollutants, diverting them to the electron-rich area via the C–O–Cu bonding bridge (Figure 8F). This study significantly deepened the understanding of the enhancement of cation-π-type DRCs through surface complexation by functionalizing with organic solid-phase ligands.
 |
Figure 8 (A) XPS spectra in Cu 2p and O 1s for CN-Cu(II)-CuAlO2. (B) EPR spectra of the fresh g-C3N4, CuAlO2, and CN-Cu(II)-CuAlO2 samples. (C) DFT calculations for the optimized structure (left) and the corresponding two-dimensional valence-electron density color-filled maps (right) of the CN-Cu(II)-CuAlO2 model in -Cu(II)-CN vision fragment and -CN vision fragment. (D) BPA degradation curves in various suspensions with H2O2 (the inset shows the corresponding kinetic curves) and TOC removal curves during BPA degradation. (E) H2O2 decomposition curves during BPA degradation in CuAlO2 and CN-Cu(II)-CuAlO2 suspensions. (F) EPR spectra for •OH and (d) HO2•/O2•− in various suspensions in the presence of H2O2 with and without pollutants. Adapted with permission from ref. [26], Copyright©2018, American Chemical Society. |
In 2019, a C–O–Cu bonding bridge was successfully constructed in the study of Cu-doped mesoporous PI nanocomposites (Cu-MP NCs) [59]. Both experimental and theoretical results showed that Cu+ and Cu2+ combined with the aromatic ring of the PI substrate through the C–O–Cu bonding bridge, creating electron-rich Cu regions and electron-poor aromatic ring regions under the influence of cation-π interactions. This specific surface MEF resulted in the adsorption and electron donation of pollutants on the electron-poor region (π-π interactions), contributing to the inhibition of H2O2 oxidation and further accelerating the electron transfer cycle from the electron-poor to the electron-rich region. Additionally, O2 was innovatively activated into O2•− in the electron-rich Cu region, further improving the energy utilization of the system. With the assistance of H2O2, Cu-MP NCs were able to rapidly degrade refractory pollutants over a wide pH range (2.6–10.4).
The aforementioned work strongly demonstrated the feasibility of constructing efficient DRCs through surface complexation using Cu species to form C–O–M bonding bridges. Based on this, other metal elements (Co, V, etc.) were introduced to construct C–O–M bonding bridges, significantly enriching the types of surface-complexed MEFs. A three-dimensional (3D) hybrid of vanadium tetrasulfide cross-linking graphene-like carbon (VSO C(π)) with π electrons was constructed to achieve a sustainable electron cycle of H2O2, DO, and pollutants at the solid-liquid micro-interface, exhibiting excellent performance in the removal of refractory pollutants [60]. The construction of C-S-V(π) and C-O-V(π) bonding bridges in VSO-C(π) triggered the directional electron transfer from C(π) to the V region, forming the surface electron polarization distribution MEF and enabling electron reduction of O2 and H2O2 in the electron-rich V region. This sustainable electron cycling process resulted in exceptional activity (~90% rhodamine (RhB) removal without H2O2 (Figure S2A) and ~98% RhB removal with H2O2 (Figure S2B) in 15 min) and adaptability to complex environments (pH and salt changes), as well as a significant reduction in resource energy consumption. Moreover, C–O–Co bonding bridges were successfully developed through surface complexation via in situ modification of Co species to graphitic carbon-CN substrates (in-situ-Co-g-C3N4) [74] (Figures S2C and S2D). Notably, the electrons within these surface DRCs were rapidly transferred through the C–O–Co bonding bridge, achieving a reaction rate ~150 times higher than g-C3N4. Additionally, using this constructed MEF, the effect of pollutant concentration on MEF performance was investigated (Figure S2E). Both high and low concentrations could affect the contact probability between the pollutant and the MEF, thereby influencing electron transport. Under suitable reaction conditions, efficient H2O2 reduction and pollutant electron donation through π-π interactions were achieved in the electron-rich Co region and the electron-poor C region on the g-C3N4 aromatic ring, respectively (Figure S2F). The above construction of non-Cu-based MEFs through surface complexation provided a promising strategy for exploring more efficient synergy with cation-π-type MEFs.
Similarly, C–N–Fe bonding bridges were systematically investigated to verify the generality of surface-complexed MEFs for efficient synergy. C–Fe–N–C bonding bridges were directly constructed in lotus-like Fe-doped PI hybrid nanosheets (lf-Fe/PI HNs) [33], generating electron-rich Fe and electron-poor C regions (Figure S3A), respectively. This novel Fe-based MEF structure could rapidly remove refractory pollutants under neutral conditions, achieving reaction rates 7 to 10-fold higher than conventional Fe-based Fenton catalysts (Figure S3B). The Fe–N–C bonding bridge served as an electron transfer and electron storage carrier between pollutants and H2O2, enabling efficient reduction of H2O2 in the electron-rich Fe region and electron donation by pollutants in the electron-poor C region, which greatly reduced the energy consumption of water treatment (Figure S3C). Furthermore, in 2022, C–N–Fe bonding bridges and numerous OVs were constructed on C3N4 substrates through self-assembly and calcination processes in FexOy-d-g-C3N4 composites [61]. This specific surface MEF enabled OVs to exhibit two types of effects on different pollutants under air-saturated conditions (Figures S3D and S3E). Hydrophilic hydroxyl pollutants primarily donated electrons to OVs through surface complexation of Fe-N bonding bridges, while H2O2 was mainly effectively reduced to •OH by obtaining electrons in OVs. In contrast, when interacting with hydrophobic pollutants, H2O2 was decomposed to O2 primarily in the Fe and OVs regions for electron donation. Therefore, this specific surface MEF exhibited higher Fenton-like efficiency for the degradation of hydroxyl-containing pollutants and hydrophobic pollutants mixed with the former than conventional Fenton catalysts (Figure S3F).
Enhanced interfacial coupling driving H2O2-assisted purification process
The cation-π electrostatic interaction plays a critical role in constructing MEF with electron polarization distributions of DRCs. This interaction can be enhanced by interfacial coupling, particularly the polar coupling process caused by nano zero valence metal species, which induces the utilization of dissolved O2, H2O, and pollutants in water assisted by H2O2. In 2019, reduction state Cu (RSC) species-doped carbon-nitrogen-oxygen polymers (RSC-CNOPs) and Cu(RSC)-O-C(π) bonding bridges were successfully fabricated by high-temperature polymerization [35]. Bidirectional electron transfer from RSC and C(π) to O (RSC→O←π) formed an electron-rich O region and an electron-poor C(π) region, resulting in ~8-fold higher free electrons in RSC-CNOPs compared to CNOPs (Figures 9A–9C). In this way, excellent pollutant degradation performance, as well as efficient utilization of pollutant energy and electrons, was achieved when the specific DRCs synergized with H2O2 (Figure 9D). Under interfacial coupling cation-π interaction, this MEF captured and utilized electrons from H2O and pollutants in the electron-poor C region and selectively reduced H2O2 in the electron-rich O region (Figure 9E).
 |
Figure 9 (A) Fourier transforms of Cu K-edge EXAFS for Cu foil, Cu2O, and RSC-CNOP. (B) Solid-state EPR of various samples. (C) The valence-electron density maps of the RSC-CNOP model fragment in the Cu-O-C calculated-plane, O-C-C(N) calculated-plane, and aromatic ring calculated-plane and ESP map of the RSC-CNOP model fragment. (D) Conversion curves of BPA in different Fenton-like suspensions (inset shows the corresponding TOC removal curves). (E) DMPO spin-trapping EPR spectra for HO2•/O2•− and •OH in the absence of H2O2, HO2•/O2•− and •OH in the presence of H2O2 in RSC-CNOP suspensions with/without pollutants. Adapted with permission from ref. [35], Copyright©2019, American Chemical Society. |
Modulating the electron distribution in the electron-rich region to construct a reduced-state-enhanced surface MEF could improve the activity of Fenton-like reactions as well as H2O2 utilization. Nanoscale zero-valent Cu doped with Cu(II) reduced graphene oxide (rGO) hybrids (nZVC-Cu(II)-rGO) was successfully fabricated via an annealing reduction process [69]. Nanoscale Cu(0) and Cu(II) generated on the rGO surface were bonded to the substrate via a C–O–Cu bonding bridge, forming an electron-rich Cu(II) region and an electron-poor C region due to cation-π interactions (Figures 10A–10C). The generation of Cu(0) strengthened the polarization of the electron-rich region on the surface MEF and greatly accelerated the electron transfer in the system, promoting the reduction of H2O2 to •OH in the electron-rich region. This surface MEF achieved ~77-fold and 13-fold higher conversion of organic pollutants than the graphene oxide (GO) system and the rGO system, respectively (Figure 10D). Pollutants in this system could significantly replace H2O2 as the electron donor and be efficiently oxidized and degraded in the electron-poor C region, with their electron energy fully utilized. Additionally, the encapsulated RSC with carbonized metal-organic framework (ZVC@CMOF) [10] and nitrogen-doped graphitic carbon substrate (Cu0@CuOx-NC) [70] were successfully developed, respectively, both achieving rapid pollutant removal and effective reduction of H2O2 (Figure 10E) and O2 (Figure 10F) in the electron-rich Cu region, greatly reducing energy consumption in water treatment. Furthermore, reduced state Co, including nano zero-valent cobalt (nZVCo) and Co2+ in co-doped silica nanospheres (mp-RSCo-SiO2 NSs), was successfully developed and connected to the SiO2 framework via the Co–O–Si bonding bridge. In Fenton-like reactions, the electron donation effect of organic pollutants was achieved through the “pollutant-Co2+/0-SiO2” pathway, which was subsequently utilized for efficient reduction of H2O2 to generate •OH. This constructed surface MEF rapidly removed a series of refractory pollutants in a wide pH range (3.1 to 10.9) through synergy with H2O2, achieving ~290-fold higher reaction rates than Co3O4. The construction of reduced-state MEF with the coexistence of zero-valent and low-valent metals provides a new strategy to improve pollutant electron utilization efficiency as well as the Fenton reaction rate.
 |
Figure 10 (A) Fourier transforms of k3-weighted EXAFS oscillations obtained at the Cu K-edge of Cu foil, Cu2O, CuO and nZVC-Cu(II)-rGO. (B) Cu 2p and C 1s of nZVC-Cu(II)-rGO. (C) Formation of active electron-poor/rich micro-regions on nZVC-Cu(II)-rGO surface. (D) 2-CP degradation curves in various suspensions with H2O2. (E) Actual and stoichiometric H2O2 consumptions and the utilization efficiency of H2O2 during 2-CP degradation in the nZVC-Cu(II)-rGO (5 wt %) suspension. (F) BMPO spin-trapping EPR spectra for HO2•/O2•− in the nZVC-Cu(II)-rGO suspensions in the absence of H2O2 with/without pollutants. Adapted with permission from ref. [69], Copyright©2019, Elsevier. |
Moreover, based on the primary cation-π DRCs catalytic system, the multi-state MEF constructed by multi-metal doping was found to strengthen interfacial coupled cation-π interactions. Han et al. [58] successfully prepared CMS-rGO NSs catalysts with Co and Mo dual electron-rich centers and C electron-poor centers by embedding Co-doped MoS2 nanomicrospheres into rGO nanosheets (Figure 11A). The EXAFS results confirmed that the formed Mo-S-C bonding bridge between CoMoS2 nanospheres and rGO nanosheets activates π electron transfer from rGO to metal centers (π → Mo and Co) (Figure 11B). Meanwhile, Co replaced Mo in the MoS2 lattice to form Mo–O–Co bonds, further increasing the electron density around Mo species due to the greater electronegativity of Mo than Co. Consequently, the novel MEF of CMS-rGO NSs consists of dual-stage electron-rich centers at Mo and Co sites with electron-deficient centers around the graphene aromatic structure. This multistage DRCs nanocatalyst demonstrated impressive reactivity for in situ generation and synchronized activation of H2O2 at different active centers, resulting in rapid and efficient pollutant degradation (Figure 11C). The reaction rate was ~21-fold higher than that of conventional Fenton catalysts. Pollutants served as electron donors throughout the reaction, and were degraded directly in the electron-poor centers of CMS-rGO NSs. Simultaneously, pollutant electrons efficiently transferred to reduce H2O2 in the electron-rich centers (Mo or Co), producing •OH for pollutant removal (Figures 11D and 11E). The discovery of multi-stage MEF provides a new strategy for enhancing interfacial coupled cation-π interactions.
 |
Figure 11 (A) Schematic illustration for the synthesis routes of CMS-rGO NSs. (B) Fourier transforms of k3-weighted EXAFS oscillations obtained at the Mo K-edge of MS, MS-rGO NSs and CMS-rGO NSs and EXAFS curve fittings of CMS-rGO NSs on [χ(R)] and Re[χR)]. (C) Decomposition curves of different dye pollutants in CMS-rGO NSs suspensions with H2O2 and kinetic curves for RhB degradation in various suspensions with H2O2. (D) BMPO spin-trapping EPR spectra for HO2•/O2•− and •OH in the CMS-rGO NSs suspensions in the absence/presence of H2O2 with/without dye pollutants. (E) Schematic for the novel Fenton-like reaction process in CMS-rGO NSs systems under Catalyst+H2O/O2+H2O2+Dye conditions. Adapted with permission from ref. [58], Copyright©2019, Elsevier. |
Metal-free MEF driving H2O2 activation process
The aforementioned work demonstrated that the synergy of H2O2 with MEF surfaces did not directly depend on the valence change of the metal element but on the strength of the polarization of electronic microregions. The introduction of metal species only served to regulate electron distribution, and therefore, metal species were not essential for MEF construction. By regulating the electron distribution on metal-free catalyst surfaces, H2O2-assisted metal-free MEFs can be developed. In 2018, non-metallic MEFs were successfully developed on the surface of 4-phenoxyphenol-functionalized rGO nanosheets (POP-rGO NSs) by surface complexation and copolymerization [36]. Experimental and theoretical studies verified the formation of electron-poor/rich microregions on the C–O–C bridge of POP-rGO NSs (Figures 12A and 12B). The efficient capture of pollutant electrons in the electron-poor C region and the effective reduction of H2O2 in the electron-rich O region were ultimately achieved on this specific metal-free MEF surface. When this metal-free MEF was synergized with H2O2, pollutants were degraded and mineralized quickly in a wide pH range, accompanied by high H2O2 utilization efficiency (Figure 12C). This work greatly expanded the types of H2O2-assisted MEFs and provided new perspectives for the construction of surface MEFs. Subsequently, in the study of 4-phenoxyphenol molecule-doped rGO nanocomposites (rGO-4-PP Nc) [75], we confirmed the formation of C–O–C bonding bridges, electron-rich O regions, and electron-poor C regions through a combination of experiments and DFT calculations. This study provided a theoretical computational basis for guiding practical synthesis and catalytic activity prediction of Fenton-like catalysts and offered an innovative perspective for the development of a new generation of metal-free MEFs by modulating electron distribution using organic polymers.
 |
Figure 12 (A) C 1s XPS spectra of POP-rGO NSs and EPR spectra of GO, rGO, POP-rGO NSs, and POP. (B) Distributions of electrostatic potential (ESP) for different graphene fragments that O atom located in different positions via DFT calculations: No O atom (represents pure graphene), O atom in the surface oxygen functional groups (represents GO or rGO (with –OH groups)), and O atom in C–O–C bonding bridge that connected the graphene and POP molecule (represents POP-rGO NSs fragment). (C) 2-CP degradation curves in various suspensions with H2O2 and actual and stoichiometric H2O2 consumptions and the utilization efficiency of H2O2 during 2-CP degradation in the POP-rGO NSs suspension. Adapted with permission from ref. [36], Copyright©2017, American Chemical Society. |
In summary, both metal substitution and surface complexation can be used to construct effective H2O2-assisted MEFs. Metal substitution is relatively simple for catalyst synthesis and surface modification, while surface complexation is more likely to form strongly enhanced MEFs. Additionally, H2O2-assisted MEFs can significantly increase the Fenton reaction rate while reducing H2O2 consumption. Given these surface MEF construction strategies, research on H2O2-assisted MEFs for water purification is thriving.
Other DRC Fenton-like processes
Based on the application of MEFs in the direction of DRC Fenton-like processes, many groups have continued to develop this theory. Since 2018, Shi’s group [76,77] has developed a series of efficient DRC catalysts through surface complexation. Zhuang et al. [76] utilized GO hydrogels to develop DRC catalysts (α-FeOOH/RGO hydrogels) with a larger specific surface area and more pores for efficient electron transfer through Fe–O–C bonding bridges by modulating α-FeOOH/rGO hydrogels with ethylene glycol (EG) (Figure S4A). Benefiting from Fe–O–C bonding bridges and defects in specific DRCs, ROS were generated even without H2O2. Moreover, electron transfer from the pollutant to the Fe region on the DRC surface was demonstrated through DFT calculations. N elements were then further introduced into the FeOOH/RGO hydrogel to enhance the formation of dual reaction centers and simultaneously reduce the reduction potential of Fe(II)/Fe(III), which increased the Fenton-like reaction rate (Figure S4B) [77]. In addition, multiple metal species were introduced to the RGO hydrogel system based on single Fe modulation to promote Fe(II)/Fe(III) redox cycling and construct stronger DRCs [78]. Similarly, in research on PVA with α-Fe2O3 [79], with Fe3O4@Fe/graphene aerogel (MGA) (Figure S4C) [80], and with Fe/S-doped aerogel (PGFe) [81], Fe–O–C bonding bridges, as well as stable DRCs, were constructed on their surface, resulting in excellent Fenton-like properties. Moreover, in research related to metal-doped substituted DRC (porous Cu-doped alumina (P-Cu-Al2O3) [82]) and metal-free DRC (biomass waste-based graphene (OG) [83]), surface DRCs with significant electron-poor/rich regions were successfully constructed, which significantly promoted the effective utilization of H2O2 and the pollutant removal reaction rate. In 2018, based on research demonstrating that σ-Cu ligands formed by phenolic hydroxyl groups and surface Cu could promote the selective degradation of phenolic compounds, Wang’s group [84] successfully constructed DRCs with a stronger electron polarization distribution by introducing the high electronegativity of Bi. The rapid degradation of pollutants and the effective utilization of H2O2 were achieved through the specific DRCs, σ-Cu-ligand, and OVs (Figure S5A). In addition, they complexed Cu-Al2O3 with g-C3N4 and C-dots to form the C–O–Cu bonding bridge to construct an enhanced MEF based on the idea of surface complexation (Figure S5B) [85]. Thus, •OH can be generated from the electron-rich region by H2O2 reduction and from the electron-poor region by H2O oxidation, respectively, realizing high H2O2 utilization. In terms of metal doping substitution, Yang’s group successfully constructed Fe–O–Zr bonding bridges [86], Fe–O–Ce bonding bridges [87], and Fe–O–Mn bonding bridges [88] by doping Fe into ZrO2 substrates, CeO2 substrates, and Mn/SiO2 substrates, respectively. All the constructed surface DRCs effectively decomposed H2O2 to remove pollutants, and OVs even promoted O2 reduction in the Fe2O3-CeO2 system. In terms of surface complexation, Chen’s group [89–92] complexed various metals on different carbon-based substrates to construct a series of different surface DRCs, enabling efficient activation of O2 to produce H2O2 under electrocatalysis, and then efficient utilization of H2O2 in different DRC regions to produce ROS for pollutant degradation. Yao’s group [93] constructed Fe-Cu alloy DRCs to achieve rapid pollutant removal. The introduction of Cu strengthened the adsorption of the Fe region on H2O2 and promoted the production of •OH. Yao’s group developed a shorter C–M bonding bridge (Figure S5C) [94] based on the C–O–M bonding bridge (Figure S5D) [95], enhancing the electron transfer between the two regions of the DRC and achieving rapid pollutant removal. In addition, the direct utilization of electronegativity differences to construct reinforced DRCs has been demonstrated in many studies, including reinforced bimetallic-organic framework DRCs [96], in situ enhanced Fe-Cu DRCs [97], and defect-rich CuVOx DRCs [98].
MEF-like configuration driving persulfate activation for water purification
Persulfates, including peroxymonosulfate (PMS) and peroxydisulfate (PDS), are derivatives of H2O2 in which the hydrogen atom is replaced by the -SO3 group. Similar to the DRC Fenton-like reaction involving H2O2, MEFs or MEF-like configurations on the catalyst surface also play a crucial role in PMS/PDS activation. Especially for the effective utilization of the electrons from ECs, it is of great significance to reduce the energy consumption and improve efficiency of PMS activation technology for water purification. Representative types of MEFs mainly include: Lattice substitution-induced MEF-like configuration, cation-π-induced MEF-like configuration and metal-free MEF-like configuration.
Lattice substitution-induced MEF-like configuration driving persulfate activation process
Lattice doping is an important means to induce MEFs. In addition to directly causing the non-uniform distribution of electrons, it often promotes the directed transfer of electrons by the formation of vacancies. Acting as electron transfer stations in most redox reactions, oxygen vacancies (OVs) are capable of both accepting and donating free electrons. For example, through lattice substitution of metal species, OVs-rich ZnFe0.8Co0.4O2.4 nanoparticles with non-uniform electron distribution surface were obtained and proven by XPS analysis and H2-TPR measurements (Figures S6A and S6B) [16]. BPA can be quickly degraded within only 4 min in the ZnFe0.8Co0.4O2.4/PMS suspension, with the reaction rate constant (k) approximately 12-fold higher than that in the ZnFe2O4/PMS system (Figure S6C). Interestingly, due to the DRC effect triggered by OVs, PMS ([O3S-OI-OII-H]) is adsorbed and trapped by surface OVs in the form of OI-OV or OII-OV. The free electrons around the OVs can be rapidly transferred to these O sites for the reduction of PMS, leading to the generation of •OH, sulfate radical (SO4•−), and H2 (Figure S6D). Meanwhile, ECs are adsorbed on metal Co sites, where their electrons are captured and transferred to the surface OVs, achieving efficient electron recycling (Figure S6E).
Sulfur vacancies (SVs), resulting from the inequivalent lattice substitution of Co for Zn in the framework of ZnS QDs,have also been found to regulate the PMS activation (Figures S7A–S7D) [99]. The Co-ZnS QDs/PMS system exhibited a significant enhancment in ECs degradation compared to the ZnS QDs/PMS system, achieving complete elimination of ECs within only 10 s (Figure S7E). For actual wastewater, the fluorescence intensity of the fulvic-like compounds decreased significantly, indicating effective removal of dissolved organic matter and a significant improvement in effluent biodegradability (Figure S7F). Combining with DFT calculations, the stable adsorption energies (Eads) of PMS on Co and SVs sites were −0.10 and −4.77 eV, respectively, indicating a preference for PMS adsorption at SVs sites. The stable Eads of pollutants on Co and SVs sites were −1.09 and −1.23 eV, respectively, suggesting that pollutants tended to adsorb at Co sites (Figure S7G). The interfacial reaction mechanism research revealed that driven by the constructed MEF-like configuration, the internal electrons of pollutants were captured by Co sites, which were then transferred to surface SVs for PMS activation. This process facilitated the efficient electron recovery, achieving water purification with high efficiency and low consumption (Figure S7H).
Cation-π-induced MEF-like configuration driving persulfate activation
Orbital interaction involving metal cation-π is an important form of electron transfer regulation. To accelerate the interfacial electron transfer of PMS for ECs degradation, cation-π-induced MEF-like configurations were constructed for PMS activation/excitation. Shi et al. [67] and Han et al. [100] constructed cation-π-induced MEF-like configurations based on the formation of C–O–M bond bridge structures by the resourcelized conversion of chicken manure into DRC catalyst, resulting in an unbalanced electron distribution on the catalyst surface, enabling PMS to trigger the sustainable electron donation of ECs and the electron gain of dissolved oxygen. As a result, the systems exhibited excellent performance in removing various ECs with PMS. The degradation rate can even be maintained above 94.3% after 10,000 cycles (Figures S8A, S8B). This MEF-like configuration can even efficiently purify actual wastewater, including the effluent from the dyeing and textile industry, pulp and paper industry and the kitchen oil wastewater, with a lower energy consumption.
Zhang et al. [15] reported a strategy through bonding atomically dispersed cobalt with nanospheric C-based graphene-like structures (SACo-NGs) to form metal cation-π structure, driving rapid and directional transfer of the electrons from ECs to PMS on the catalyst surface. It is found that Co-π structures (Co2+-N-Cπ) play a key role in the efficient activation of PMS, resulting in the rapid removal of ECs. During the reaction, pollutants can donate electrons for the system through π-π interaction accompanied by the direct oxidative degradation of pollutants. The obtained electrons are quickly transferred to the atomically dispersed cobalt sites through the formed cation-π structure, which promotes the activation of PMS (Figure S8C). This work demonstrates that single-atom catalysts (SACs) with adjustable coordination environments of metal sites are more conducive to regulating electronic structures and forming cation-π MEF-like configurations on the catalyst surface. Zhao et al. [68] found that the inert element Zn can be turned into an active single-atom catalyst (SA-Zn-NC) by forming an atomic Zn-N4 coordination structure with cation-π structure on the catalyst surface. During the reaction, electrons are spontaneously transferred from electron-rich contaminants and PMS to single Zn atoms, driving the reduction of DO to O2•− and 1O2 species over the electron-attracting single-atomic Zn-N4 site, thereby achieving the efficient elimination of ECs through multi-pathways including self-oxidation and attack by ROSs. In addition, Li et al. [101] demonstrated that a single-atom Cu catalyst with a low N configuration (Cu-N2) is more conducive to PDS activation than the saturated Cu-N4 configuration (Figure 13A). Benefiting from the generation of Cu (III) derived from unsaturated Cu-N2 sites, CuSA-NC exhibited a high metal utilization rate, selectivity for ECs degradation, and resistance to matrix interference (Figure 13B). With the activation of PDS, 2,4-dichlorophenol (2,4-DCP) could be completely removed within 30 min in the CuSA-NC system, whereas only 15%–20% of 2,4-DCP was removed in the Cu2O, CuO, and metal-free NC systems (Figure 13C). The results of continuous-flow experiments further demonstrate the good durability of CuSA-NC, making it promising for practical environmental remediation (Figure 13D).
 |
Figure 13 (A) Structural characterization of CuSA-NC catalyst. (B) Optimized atomic Cu-N4 and Cu-N2 configuration, partial density of states (PDOS) for Cu-N4 and Cu-N2 sites, and the reaction pathway of PDS activation and 2,4-DCP oxidation on CuSA-NC. (C) ECs degradation performance in different systems. (D) 2,4-DCP concentration in the effluent from a continuous-flow reactor consisting of the CuSA-NC-filled column (inset: diagram of the continuous-flow reactor). Adapted with permission from ref. [101], Copyright©2022, American Chemical Society. |
PMS as inducer driving electron donation of pollutants over metal-free MEF-like configuration
The studies above indicate that MEF-like configurations on the catalyst surface play an important role in regulating the selective reduction of PMS without relying on the valence changes of metal species. This finding sheds light on the potential for regulating non-equilibrium electronic structures in metal-free catalysts.
Polyimide (PI) contains a significant number of delocalized π electrons, making it an ideal metal-free catalyst for PMS activation [102,103]. Cao et al. [102] converted PI into L-ascorbic acid doped carbon-nitrogen-oxygen polymer nanoflowers (OVc-CNOP Nfs) with surface MEF structures through hydrothermal cascade pyrolysis processes (Figure S9A). The doped O atoms induced bidirectional transfer of electrons on aromatic rings through C–O–C bonding [C(π)→O←C(π)] to form the reinforced O electron-rich centers and electron-poor C(π) centers (Figure S9B), triggering the sustainable electron donation of ECs and high-efficient PMS activation. As the results, the reaction rate of the OVc-CNOP Nfs system was approximately 10-fold higher than that of the unmodified CNOP system, even the refractory endocrine interferon BPA can be completely removed in just 1 min (Figures S9C and S9D). This work provides a primary reference for the construction of surface metal-free MEFs to drive PMS activation.
Based on the construction strategy of metal-free MEF-like configuration, Li et al. [14] developed the multiporous oxygen-rich carbon-nitrogen nanosheets (OLAA-CN NSs) through a staged temperature-programmed calcination of l-ascorbic acid (LAA)-modified dicyandiamide precursor (Figure 14A). It is found that the oxygen species from L-ascorbic acid (OLAA) are introduced into the graphene-like basic matrix and replace partial N atoms to form the C-O-C-R structure, leading to the non-uniform distribution of electrons on the catalyst surface, and the formation of surface MEF-like structures according to a series of characterization techniques (Figure 14B). As a result, OLAA-CN NSs exhibit excellent performance for ECs removal in the presence of PMS and DO. Notably, BPA, CIP, and 2-CP can be completely degraded within only 1 min (Figure 14C). An innovative discovery was that PMS mainly played the role of an inducer to drive the donation of the electrons from ECs and these electrons are finally utilized by dissolved oxygen through the interface process (Figure 14D). This is a key progress for reducing resource and energy consumption through the construction of metal-free MEF-like configuration in advanced wastewater treatment.
 |
Figure 14 (A) SEM and TEM images of OLAA-CN NSs. (B) Characterization of OLAA-CN NSs. (C) Decomposition curves of different ECs in OLAA-CN NSs/PMS suspensions. (D) Interface reaction mechanism of OLAA-CN NSs process for pollutant removal under initiation of PMS. Adapted with permission from ref. [14], Copyright©2022, Elsevier. |
Other MEF-like configuration driving persulfate activation
The construction of MEF-like structures is conducive to generating surface potential differences that trigger the electron gain and loss processes of persulfate and pollutants in the two polar centers, enabling the synchronous occurrence of oxidation and reduction reactions. However, implementing the construction of this potential difference on the surface of many inert materials is challenging.
It has been discovered that the photo-assisted process enhances the potential difference, facilitating rapid persulfate activation, efficient ECs degradation, and resolving the issue of photogenerated electron-hole recombination in photocatalysis. Wang et al. [104] developed a nanoscale iron molybdate (Fe2(MoO4)3) catalyst by introducing metal atoms into molybdate nanoparticles to induce the unbalanced electron distribution on the catalyst surface, thereby constructing the MEF-like structures. Based on the Fe2(MoO4)3 system, BPA (10 ppm) can be rapidly degraded through the heterogeneous photo-combined PDS activation (HPPA) process, and the degradation rate increased 98-fold compared with that without light (Figures S10A, S10B). Combined with the electrochemical characterization, the instantaneous photocurrent of the Fe2(MoO4)3/PDS/light system was increased 5 fold compared to that of the original Fe2(MoO4)3 electrode system (Figure S10C), accompanied by the smallest arc radius in the EIS Nyquist plot (Figure S10D). During the HPPA reaction, PDS and DO molecules are rapidly activated and generated SO4•−/•OH and O2•− radicals by capturing photogenerated electrons at CB (electron-rich centers) and driving the electron-donor of ECs around electron-poor centers at VB (holes) through π-π interaction. This synergistic process not only inhibited the electron-hole recombination but also enabled rapid dual-pathway degradation of pollutants through free radical attack and hole oxidation (Figure S10E) [104]. In actual systems, the formation of MEF-like structures on the catalyst promotes the utilization of abundant DO and pollutant energy in water, significantly reducing PDS consumption and achieving efficient water purification triggered by trace PDS. This approach is cost-effective and feasible. Other interesting studies [105,106] reported that the selective regulation of photo-assisted reaction process can be achieved by introducing metal atoms into the catalyst to cause the change of the surface electronic structure, leading to efficient PMS activation and ECs degradation.
MEF-like configuration driving photocatalysis for water purification
Semiconductor photocatalysis is a promising advanced oxidation technology for water treatment that utilizes clean solar energy [107–109]. For photocatalysis, lattice substitution, vacancy introduction, and functional group grafting are effective methods to polarize surface charge distribution for MEF construction. MEFs not only promotes the separation and transfer of e–/h+ but also strengthens the electron donation from adsorbed pollutants, which modifies the energy band structure of the catalyst. Some studies have proposed the concept of internal electric field (IEF) formed by polarizing the charge distribution in local microregions. The construction mechanism is similar to that of MEF, so this type of IEF can be considered equivalent to MEF. It should be noted that the IEF formed by a heterogeneous structure on large-scale interfaces differs from the MEF, as discussed in this review.
A dual-oxygen group (C–O–C and C=O) doped CN (ACN*) was synthesized using N lattice substitution for the photocatalytic degradation of pollutants (Figure 15A) [110]. Due to the strong electronegativity of O elements, the O atoms in the C–O–C and C=O groups exhibited higher valence-electron densities than the C and N atoms in the g-C3N4 skeleton. This constructed the surface MEFs in ACN* and provided a direct surface driving force to promote the separation and transfer of e‒/h+ (Figure 15B). More importantly, the electron-poor area could strengthen the adsorption of aromatic pollutants and promote direct electron delocalization from the pollutant to the catalyst. The adsorbed pollutants have been integrated with ACN* and regulated the band structure of ACN*. The photocurrent intensity of ACN* with pollutant adsorption was 2–3 times higher than that of ACN*. Moreover, more O2•− was generated with the assistance of pollutant adsorption for pollutant degradation (Figures 15C–15E).
 |
Figure 15 (A) Schematic illustration of preparation for ACN*. (B) The two-dimensional valence-electron density color-filled maps of CN, ACN, and ACN* (multi-directional spliced diagram). (C) BPA degradation experiments. (D) Transient photocurrent responses of ACN* adsorbed different pollutants. (E) BMPO spin-trapping EPR spectra for O2•−. (F) Electrostatic potential (ESP) maps of the g-C3N4 model compounds, i.e., monomer (leftmost column), dimer (second column), trimer (third column) and tetramer (rightmost column). (G) Solid EPR spectra of BCN and LCN before and after adsorbing different pollutants. (H) UV-vis DRS spectra of BPA, BCN and LCN under different condition. (I) Transient photocurrent response spectra of LCN before and after adsorbing different pollutants in air/Ar condition. (J) BMPO spin-trapping EPR spectra for BMPO-O2•− in the HTCN system. (A)–(E) Adapted with permission from ref. [110], Copyright©2019, Elsevier; (F)–(I) adapted with permission from ref. [111], Copyright©2021, Elsevier; (J) adapted with permission from ref. [112], Copyright©2022, American Chemical Society. |
Additionally, defects of catalysts tend to intensify the uneven distribution of electrons and promote the construction of surface MEFs. We prepared a partially low-polymerized g-C3N4 (LCN) with more C-NHx exposure by attacking oxygen-doped g-C3N4 with high-temperature vapor [111]. Increased C-NHx exposure reinforced the charge polarization distribution to construct surface MEFs. In this case, the electron-rich area was centered at C-NHx, while the electron-poor area was distributed at the C-N skeleton. The surface MEFs reinforced the interaction between pollutants and catalysts, further promoting electron delocalization from the pollutant to the catalyst (Figures 15F, 15G). The optical absorption intensity of LCNBPA was further extended in the visible-light range compared to that of LCN (Figure 15H), and the separation and transfer efficiency of e‒/h+ (Figure 15I) were also significantly improved. These results indicated that the adsorbed pollutant modified the energy band structure and could be directly excited by light irradiation, accompanied by the cleavage of pollutant molecules. Additionally, owing to surface MEFs, more O2•− was generated in g-C3N4 with C vacancy defects (HTCN) by the modification of pollutant adsorption (Figure 15J). This finding indicates that the energy of the pollutant molecule was utilized by the photocatalytic system [112].
Lattice doping can also be performed on inorganic photocatalysts. Interstitial carbon-doped Bi3O4Cl (C-doped Bi3O4Cl) nanosheets were synthesized with glucose as a carbon source, exhibiting a 126-fold increase in IEF between layers due to carbon introduction into Bi3O4Cl [113]. The interlayer IEF drove electrons and holes to transfer from e–/h+ separation sites to the [Bi3O4] and [Cl] slices for surface reactions (Figure 16A). Our group reported a layered structural BiOBr doped with sulfur (S-BiOBr) [47], consisting of covalent [Bi2O2S]2+ layers and exchangeable bromide ions [Br2]2–. Due to the lower electronegativity of the S atom than that of the Br atom, a strong interlayer IEF was constructed between the covalent [Bi2O2S]2+ layers and exchangeable bromide ions [Br2]2–, which acted as a driving force for charge carrier separation and transport (Figure 16B). Notably, after 30–60 s of illumination, CIP could be continuously degraded in the S-BiOBr suspension in the dark condition (Figure 16C), and the signal of BMPO-O2•− in the S-BiOBr suspension with CIP adsorption was significantly enhanced (Figure 16D). With the assistance of the enhanced IEF, electrons arising from the R• radical could be sequentially transferred to the S-BiOBr, where O2 was reduced to O2•−, acting as a major contributor to CIP oxidation in the dark condition.
 |
Figure 16 (A) Schematic illustration of the separation and migration of electrons and holes in the bulk of the pure and C-doped Bi3O4Cl. (B) Charge distribution of S-BiOBr. (C) Removal efficiency of CIP by S-BiOBr-3 in the dark after 30, 60, and 120 s of illumination. (D) EPR spectra for BMPO spin trapping of O2•− and R• in S-BiOBr with/without CIP adsorption. (A) Adapted with permission from ref. [113], Copyright©2016, Wiley-VCH; (B)–(D) adapted with permission from ref. [47], Copyright©2022, Elsevier. |
Grafting electron-withdrawing/donating moieties onto organic photocatalysts via molecular copolymerization can promote the polarization of charges in organic photocatalysts due to the unique flexibility of organic semiconductors [114]. Molecular copolymerization differs from conventional semiconductor heterojunctions, which builds up an IEF at the two-phase interface [115]. Similar to surface MEFs, countless charge polarization regions can be formed on a smaller scale and evenly distributed in catalysts between the electron-donating and accepting moieties. Fan et al. [116] incorporated aromatic rings into g-C3N4 by connecting them with 3s-triazine units, effectively facilitating charge transfer and separation. Chen et al. [117] also prepared an aromatic ring-terminated g-C3N4 (ARCNSs) via a one-step copolymerization approach with quinazoline-2,4-diamine as the precursor of the terminated aromatic rings, which extended the electron delocalization of g-C3N4. Furthermore, Chu et al. [118] incorporated an electron-deficient pyromellitic dianhydride (PMDA) monomer into the skeleton of g-C3N4 (Figure S11A). The two electron-withdrawing anhydride groups in PMDA not only separated the reduction and oxidation sites but also promoted a shift of the negative region from melem to the O atoms of PMDA, which enhanced the separation and transfer of h+/e– for MO degradation (Figures S11B–S11D). Perylene plane series materials are also ideal photocatalysts for adjusting the delocalized π-electrons by introducing electron-withdrawing groups [119]. Perylenetetracarboxylic acid nanosheets (PTA) were synthesized via a simple hydrolysis reassembly of PTCDA. The π orbital of the PTCDA molecule was evenly distributed on the entire molecular plane, whereas a nodal plane appeared on the π orbital of the PTA molecule between the perylene plane and the carboxyl group (Figure S11E). The electron cloud mainly centered at the carbonyl oxygen in PTA, and the excited charge density difference of PTA was 4.53 × 10−3 eV Å−3, which was significantly stronger than that of PTCDA (0.97 × 10−3 eV Å−3) (Figure S11F). The intensity of the electric field between the perylene plane and the terminal carboxyl group in PTA was approximately 10.3 times that of PTCDA, efficiently producing anisotropic charge migration to promote surface reactions (Figures S11G, S11H). In fact, the IEF on two-dimensional layered materials can be classified as a surface MEF. However, most of the above studies focused on hydrogen or H2O2 production by utilizing the IEF/MEF for charge transfer, neglecting the role of electron-rich and the electron-poor areas. The interaction between the pollutant and electron-poor area and the utilization of the pollutant were not considered during the pollutant degradation process. Therefore, the further development and comprehensive utilization of surface MEFs have great prospects in the field of photocatalytic water treatment.
MEF driving water self-purification by internal energy utilization
MEF driving surface ECs conversion and oxygen reduction
Previous research has confirmed that constructing MEFs by modulating the surface structure of catalysts can drive the energy utilization of organic pollutants for water purification. However, efficient pollution degradation in such a process still requires a small amount of oxidants or additional energy assistance. Additionally, we have found that the electrostatic force of surface MEFs on the catalyst is crucial for the energy utilization of organic pollutants. Inspired by this discovery, we have hypothesized that further directional modulation of the catalyst surface structure could enhance the MEF potential difference and strengthen the adsorption of surface molecules on MEFs. This, in turn, could weaken their chemical bonding energy through strong surface orbital interactions. Under such conditions, it might be possible to trigger interfacial chain reactions using weak electron acceptors such as O2 to achieve a self-purification process of wastewater.
Based on this assumption, a novel and efficient surface MEF was created using the lattice substitution method on 3D pomegranate-grain-like nanoparticle-integrated molybdenum (Mo) lattice-doped zinc sulfide (ZnS) composites (MZS-1) [25]. The (002) crystalline plane of MoS2 was observed, and the Raman peaks for Zn–S bond vibration showed a blue shift after introducing Mo in ZnS (Figures 17A, 17B), confirming the formation of Mo–S–Zn bonding bridges in MZS-1. Doping Mo in the lattice of ZnS induced electron transfer from Zn to Mo through the Mo–S–Zn bonding bridge, driven by their electronegativity difference. Thus, the non-equilibrium distribution of electrons on the MZS-1 surface was enhanced, resulting in a strong surface MEF (Figure 17C). Notably, the MZS-1 system achieved over 80% catalytic efficiency for RhB even after continuous operation for 168 h (Figure 17D). ECs were easily chemisorbed on surface MEF to form a strong interfacial interaction during the reaction. They then donated electrons to the catalyst surface to be oxidized by the delocalized π-π conjugation effect. Additionally, the complexation between DO and MEF effectively reduced the activation energy barrier of O2, making it easier to activate O2 into ROS (•OH, O2•− and 1O2) by utilizing delocalized electrons and energy in the electron-rich Mo area (Figure 17E). Thus, the entire RhB elimination process involved two independent processes (Figure 17F): direct oxidation due to the electron delocalization effect and oxidative degradation by ROS. This study achieved sustainable DO activation for water purification by utilizing the energy of pollutants through surface MEF, which provides new insight into developing low-energy consumption and sustainable wastewater treatment technology.
 |
Figure 17 (A) XRD patterns of ZnS and MZS-1. (B) Raman spectra of ZnS, MoS2 and MZS-1. (C) Local potential of MZS-1 by DFT calculation. (D) Stability test of MZS-1 reactor in degrading RhB. (E) EPR spectra for BMPO-•OH, BMPO-O2•− and TEMPO-1O2 in various suspensions. (F) Schematic illustration of sustainable microactivation of DO and ECs conversion on the MZS-1 surface. Adapted with permission from ref. [25], licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
On this basis, Cao et al. [71] further enhanced the nonequilibrium electron distribution on an nZVI/FeOx/FeNy-anchored NC composite (nZVI/FeN5.5O7.2/C) surface by constructing cation-π bonds. Based on EXAFS and EPR results (Figures S12A, S12B), iron species were produced in situ in the NC composites through Fe–O–C and Fe–N bonding bridges, which transferred electrons from C(π) and N to Fe simultaneously (N → Fe ← π) through these bonding bridges. This resulted in the formation of a strengthened surface MEF comprising electron-rich Fe microcenters (ERCs) and electron-poor C(π)/N microcenters (EPCs) on nZVI/FeN5.5O7.2/C. During the reaction, the surface-adsorbed ECs formed an enhanced π-π molecular orbital interaction with EPCs, followed by electron delocalization, transferring delocalized electrons to ERCs through cation-π bonds (Figure S12C), and being oxidized, leading to rapid microreduction of O2 into O2•− around ERCs of MEF (Figure S12D). Based on these enhanced MEF surface characteristics, nZVI/FeN5.5O7.2/C showed exceptionally high activity and efficiency (62%–100%) for degrading both dyes (RhB and methylene blue (MB)) and refractory pollutants (BPA, CIP, and 2-CP) within 2–60 min at room temperature and atmospheric pressure (Figures S12E, S12F). Notably, this process was free from interference by NOM in sand filter water, indicating the potential application prospects of nZVI/FeN5.5O7.2/C (Figure S12G). This finding provides new insight for addressing the key limitation of the classical wet air oxidation process through the MEF-type catalytic system.
The aforementioned studies confirm that the electrostatic force of surface MEFs on a catalyst is key to the energy utilization of organic pollutants. Han et al. [58] creatively constructed a dual-stage MEF structure consisting of a dual-stage electron-poor and electron-rich microregion on the CMS-rGO NSs surface, generating particularly strong electrostatic forces. This unique structure enabled CMS-rGO NSs to possess a wide working pH range and approximately 75%–100% degradation efficiency for RhB, MB, methyl orange (MO), and AO7 without adding any other substance or assistance to the CMS-rGO NSs suspension (Figures S13A, S13B). Moreover, 98% RhB removal was achieved within 60 min, even after 7 successive cycle runs (Figure S13C), indicating the excellent stability of CMS-rGO NSs. By utilizing strong surface MEFs, H2O was readily adsorbed at the electron-deficient center and delocalized electrons to the electron-rich Mo/Co centers, where it was oxidized into •OH. These delocalized electrons could also be trapped by O2 to form HO2•−/O2•− and H2O2 at the electron-rich Mo/Co centers. Additionally, the ECs could replace the adsorbed H2O and form a surface-confined non-equilibrium electron orbital with the electron-deficient graphene aromatic structure area of MEF through strong π-π molecular orbital interactions. Consequently, the adsorbed pollutants could directly delocalize electrons to the electron-rich Mo/Co centers by strong electrostatic force, continuously reducing O2 (O2 → O2•− → H2O2 → •OH) for ECs degradation. In the process, efficient pollutant destruction was achieved through two pathways (Figure S13D): direct degradation by MEF and •OH attack. This discovery provides a new strategy for H2O2 generation-activation and ECs degradation by constructing MEFs on the catalyst surface.
These studies demonstrated that a strong surface MEF enables the utilization of pollutant electrons with the assistance of O2. Typically, these MEFs were constructed in one or two phases through single or double chemical bonding bridges. However, if the electrostatic force of the surface MEF could be further enhanced by constructing a heterogeneous structure with multistage cation-π interactions, the destruction and mineralization of ECs could be achieved through a self-purification process on the catalyst surface. Using surface complexation and electron polarization theory, an iron-based composite catalyst (Fe0-FeyCz/Fex-GZIF-8-rGO) was successfully developed (Figure 18A) [34]. The series of characterization results confirmed that the Fe-O-C, Fe-N, and C-O-Zn-N chemical bond bridges were formed among the three phases (Fe0-FeyCz/Fex, GZIF-8, and rGO) of Fe0-FeyCz/Fex-GZIF-8-rGO, resulting in the formation of strong (Fe or Zn)-π electrostatic force. Therefore, a dual-stage MEF consisting of the electron-rich areas around Fe species and the electron-deficient areas around GZIF-8/rGO was formed on the catalyst surface with a surface potential energy of 310.97−663.96 kJ mol−1 (Figure 18B). In the Fe0-FeyCz/Fex-GZIF-8-rGO air-saturated suspension, 27.4%−67.5% total organic carbon (TOC) removal of various ECs was achieved within 180 min without additional energy assistance (Figure 18C). The same catalytic efficiency as that under the air-saturated condition was achieved under the N2-Fe3+ condition, but not in the N2 and Cu2+ conditions (Figure 18D). The results indicated that the surface MEF could complex the ECs by forming strong π-π and H-bonding molecular orbital interactions, thereby reducing their chemical bonding energy to ~0.7 eV and delocalizing their electrons to the catalyst surface, leading to the surface cleavage of the adsorbed ECs. Additionally, under the action of the strong surface MEF, O2/Fe3+ was confined to adsorb at the electron-rich Fe species area and trap delocalized electrons to form O2•−/Fe2+ (Figure 18E), triggering the hydrolytic process of adsorbed ECs with H2O, continuously converting H2O into •OH (Figure 18F) for water purification. This work has opened a new avenue for the development of sustainable wastewater self-purification treatment technologies that utilize pollutant energy driven by surface MEF.
 |
Figure 18 (A) Schematic illustration of the preparation of Fe0-FeyCz/Fex-GZIF-8-rGO. (B) ESP distribution for different structural model fragments of Fe0-FeyCz/Fex-GZIF-8-rGO. (C) TOC removal of various ECs. (D) BPA degradation in the aqueous suspension of Fe0-FeyCz/Fex-GZIF-8-rGO under different reaction conditions specified in the panel. (E) BMPO spin-trapping EPR spectra for O2•− in air-saturated methanol suspensions with/without BPA. (F) BMPO spin-trapping EPR spectra for •OH in the air-saturated D2O suspensions with/without BPA. Adapted with permission from ref. [34], licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Licence. |
MEF driving the selective preferential removal of ECs from wastewater
Natural organic matter (NOM), a significant source of dissolved organic carbon (DOC) in water, negatively impacts water purification. However, removing NOM using current water treatment technologies remains a scientific challenge. After confirming the stability and high catalytic efficiency of MEF on Fe0-FeyCz/Fex-GZIF-8-rGO, humic acid (HA) (10 mg/L) was also found to be highly degraded over Fe0-FeyCz/Fex-GZIF-8-rGO (Figure S14A) [120]. However, the mineralization of HA underwent three stages within 480 min (Figure S14B). It was found that HA complexed with the electron-poor rGO/GZIF-8 area through π-π and H-bonding molecular orbital interactions, delocalizing their electrons to electron-rich Fe species areas through cation-π bonding bridges. This process results in complete cleavage of HA into a few aromatic ring compounds and a large number of alcohols and alkanes within 120 min. After 120 min, the generated alkane adsorbs on alcohol to form a strongly polarized alkane-alcohol complex, including electron-rich hydroxyl group areas in alcohol and electron-poor areas in alkane (Figures S14C, S14D). Consequently, the electron-rich hydroxyl group area in alcohol tended to adsorb at the electron-poor rGO/GZIF-8 area to form a strong H-bonding interaction, promoting electrons from alkane to delocalize directly to the electron-rich Fe species area of MEF. This process accelerates interfacial electron cycle transfer (Figure S14E). The delocalized electrons trapped by O2 at the electron-rich Fe area of MEF trigger the alkane’s surface cleavage and hydrolytic process, leading to more •OH generation for reactant degradation (Figure S14F). This finding enhances our understanding of the significance of the surface complex characteristic of surface MEF and provides new insights into complex mixture-containing water purification utilizing an O2-assisted MEF catalytic system.
Due to the competition of mg-level DOC, the complete removal of ECs in water remains a significant international challenge. It was reported that DOC can adsorb ECs through its rich functional groups to form a polymeride [121]. Inspired by the interfacial interaction mechanism between surface MEF and the polarized alkane-alcohol complex, the preferential destruction of ECs from DOC-containing water was achieved by investigating the interaction of ECs with HA/DOC in synthetic and real wastewater under the action of MEF [29]. As depicted in Figures 19A and 19B, compared with the Fe0-FeyCz/Fex-GZIF-8-rGO suspension, the degradation efficiencies of various ECs increased in the Fe0-FeyCz/Fex-GZIF-8-rGO/HA suspension, and a higher degradation efficiency was observed in municipal wastewater and raw drinking water (Figures 19C, 19D). According to the three-dimensional excitation/emission matrix and EPR analysis, and DFT calculation results, we first reported that ECs tended to form a polar HA/DOC-micropollutant complex with HA in synthetic wastewater through π-π electrostatic force (Figure 19E), consisting of an electron-poor aromatic structure area of the ECs and an electron-rich phenolic group area of HA in the complex. During the reaction, a strong orbital interaction occurred between the electron-rich phenolic group area of HA in the HA/DOC-ECs complex and the electron-poor rGO/GZIF-8 area of Fe0-FeyCz/Fex-GZIF-8-rGO, forming a surface-confined nonequilibrium electron orbital with MEF. This surface-confined nonequilibrium electron orbital greatly enhanced the electrostatic interaction between the complex and MEF. Consequently, due to the enhanced electrostatic force, the ECs in the complex preferentially transferred more electrons across the HA/DOC media to the electron-rich Fe area (Figure 19F) and were oxidatively cleaved, thereby enhancing the electron transfer between MEF and the HA/DOC-ECs complex. With the delocalized electrons continuously trapped by O2 (Figure 19G), the surface cleavage and hydrolysis processes of ECs in the HA/DOC-ECs complex were carried out at the electron-poor rGO/GZIF-8 area of MEF, converting more H2O into •OH (Figure 19H). Therefore, the preferential destruction and mineralization of ECs in real water were achieved through the above chain reaction process. This work developed a novel catalytic system that achieved the preferential degradation of ECs through a self-purification process by utilizing MEF on the catalyst surface, which is very promising for future combined ECs-water purification.
 |
Figure 19 Degradation curves of various ECs (A) without HA and (B) with HA in ultrapure water. Degradation curves of BPA, DP and ATZ in (C) municipal wastewater and (D) raw drinking water suspension. (E) Highest occupied molecular orbital (HOMO) for DP, BPA and ATZ on the HA model fragments with isovalue of 0.015 bohr−3/2. (F) Solid EPR spectra and chronoamperometry curves of various samples. BMPO spin-trapping EPR spectra for (G) O2•− and (H) •OH with/without DP in the Fe0-FeyCz/Fex-GZIF-8-rGO/HA air-saturated aqueous suspensions. Reaction conditions: (A), (B) catalyst concentration 0.6 g/L, initial ECs concentration 3.0 mg/L, initial HA concentration 10.0 mg/L, temperature 35°C; (C), (D) catalyst concentration 0.6 g/L, initial ECs concentration 3.0 mg/L, temperature 35°C. Adapted with permission from ref. [29], Copyright©2022, American Chemical Society. |
In addition, many researchers have devoted significant effort to the self-purification of ECs by modulating the surface structure of catalysts to drive O2 activation. Gu et al. [122] constructed a diatomic site on a dual-adjacent Fe atom-doped MnO2 catalyst. The surface structural properties enabled CO to be confined and adsorbed on the Mn site of MnO2 (Figure S15A). Furthermore, the surface dual-adjacent Fe atoms were able to absorb O2 to form a strong chemical bond of Fe(O = O)Fe. The feedback π electrons of the Fe d-orbital were then transferred to the vacant orbital of O2, reducing the chemical bond energy of O2. Therefore, the O atom in Fe(O = O)Fe tended to bond with the carbon atom in CO and then desorated to form CO2, with an activation barrier of only 0.17 eV. Gong et al. [123] developed a Zn-carbon nanotubes (CNTs) composite by directly sintering a mixture of zinc and CNTs. During the reaction, Zn was found to donate electrons to the CNT area and was oxidized into ZnO. Meanwhile, the surface-adsorbed O2 was driven by the potential drive of the internal electrolysis of Zn to capture electrons and undergo a two-electron conversion process on CNTs, producing a large amount of H2O2 for wastewater treatment without additional assistance. Lyu et al. [124] reported a novel catalyst consisting of Pd subnanoparticles encapsulated by hierarchical TS-1 (HTS-1) for the selective oxidation of benzyl alcohol. In addition to the benzoic acid chemically adsorbed on Pd sites, some H2 and O2 tended to strongly interact with Pd sites and were dissociated to form reactive intermediates (MARI) containing a single H/O atom (H* and O*). More O2 was chemically adsorbed on the Ti sites and formed MARI containing an intact O−O bond (OO**), which was then converted into H2O2 with the adsorbed intermediate H*. The generated H2O2 subsequently complexed with Ti4+ sites to form Ti-OOH active species for the efficient oxidation of benzyl alcohol. Li et al. [125] reported that Co3O4 complexed with carbon (C-Co3O4) caused a disorder on the surface lattice of Co3O4, constructing a large number of exposed active sites of electron-rich OVs and Co3+ on the catalyst surface. Formaldehyde (HCHO) was readily chemisorbed on the Co3+ sites through a strong interaction and was converted into dioxymethylene (Figure S15B). Meanwhile, O2 and H2O were able to capture electrons from the electron-rich OVs area to effectively form activated O2 and OH for the continuous mineralization of surface-adsorbed dioxymethylene. The above research achieved the self-purification of pollutants by modulating the surface structure of catalysts to drive O2 activation, which was a little bit similar to the surface MEF effect. Although the researchers did not summarize the characteristics and role of the surface structures of these types of catalysts in the articles, these findings provided significant inspiration for the successful design and construction of MEF on the catalyst surfaces.
Conclusions and outlooks
MEFs constructed on the surface of catalysts using various methods have been widely used to drive pollutant degradation in various technical fields, including Fenton processes, photocatalysis, PMS activation, catalytic ozone oxidation, and water self-purification through internal energy utilization. This has significantly enhanced efficiency and reduced consumption during the degradation of ECs. In particular, the advent of MEF-based self-purification technology has greatly reduced the energy consumption of water treatment in response to the global goal of carbon neutrality. Moreover, MEF-containing catalysts often exhibit high structural stability even after multiple consecutive cycles of pollutant degradation, as confirmed by results from inductively coupled plasma mass spectrometry (ICP-MS), X-ray diffraction (XRD), XPS, and 57Fe Mössbauer spectra [34,60,70]. Recently, electrochemical methods have been used to test the stability of MEF, with results showing that the corrosion current density of the MEF-containing catalyst decreased from 10.99 to 4.67 μA cm−2 after reacting with pollutants, indicating corrosion inhibition. In contrast, the conventional Fe-C catalyst exhibited a significant increase in this value from 20.82 to 120 μA cm−2 [120]. These results confirm that the surface MEF stability is significantly strengthened due to the cation-π interaction and the electron-donating effect of pollutants during the reaction. This is the key to achieving continuous and efficient water purification using MEF-containing catalysts. This technology is revolutionary and forward-looking, with great development potential, providing a fast track for the development of low-energy water treatment technology. It is also of great scientific significance and practical value in treating compound micro-polluted water by achieving preferential and selective removal of ECs through precise modulation of surface MEF.
In the future, the development of MEF faces significant challenges, particularly in terms of adaptability to complex environmental media and expansion into multidisciplinary fields. The primary development directions of MEF are: (1) development of an accurate construction technology for MEF based on the REDOX potential of reactants. At present, the characteristic construction of MEF has been realized for the degradation of some types of ECs, such as antibiotics, endocrine interferons, pesticides, and synthetic dyes. However, it remains challenging to construct the REDOX potential accurately based on the specific environmental factors involved in the reaction, which is the bottleneck that needs to be overcome in the future. (2) Precise regulation and modification of multistage MEF at the atomic orbital level. Currently, research on regulating and constructing single-stage MEF is simple and productive. However, pollutants in actual water environments and wastewater are diverse and complex, requiring the construction of multistage MEF to meet the needs of actual complex pollution. (3) Research on the impact of complex environmental media, such as salt, acid-alkali, temperature, and suspended nanoparticles, on MEF and the development of innovative avoidance strategies. This is necessary to address actual water treatment needs. (4) Application development outside the field of water purification, including energy, agriculture, medical technology, and intelligent manufacturing. The application of MEF is not limited to the environmental field, as it represents a fundamental theory of surface electron polarization distribution, which is the cornerstone of regulating ordered molecular reactions and can contribute to the development of multidisciplinary fields.
Data availability
The data are available from corresponding authors upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (52350005, 52122009 and 51838005), the Introduced Innovative R&D Team Project under the “Pearl River Talent Recruitment Program” of Guangdong Province (2019ZT08L387)
Author contributions
L.L. designed the structure of this review, wrote the sections of Abstract, Introduction, Construction and properties of surface MEF on catalyst, and Conclusions and outlooks, and revised and edited the full text. He was responsible for the coherence of the full text. Y.W. wrote the sections of Construction and properties of surface MEF on catalyst, and MEF driving water self-purification by internal energy utilization. C.L. wrote the section of MEF driving DRC Fenton-like process for water purification. F.L. wrote the section of MEF-like configuration driving photocatalysis for water purification. W.C. wrote the section of MEF-Like configuration driving persulfate activation for water purification. Y.S. was responsible for the drawing and presentation of visualizations. C.H. incubated the idea for the topic, designs the structure of this review, and directed the writing of the full parts. C.H. was responsible for the review and revision of the full text.
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
The supporting information is available online at https://doi.org/10.1360/nso/20230017.
References
- Hannah DM, Lynch I, Mao F, et al. Water and sanitation for all in a pandemic. Nat Sustain 2020; 3: 773–775 [Google Scholar]
- Gomes IB, Maillard JY, Simões LC, et al. Emerging contaminants affect the microbiome of water systems—strategies for their mitigation. npj Clean Water 2020; 3: 39. [Article] [Google Scholar]
- Alvarez PJJ, Chan CK, Elimelech M, et al. Emerging opportunities for nanotechnology to enhance water security. Nat Nanotech 2018; 13: 634-641. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379: 2151–2161 [CrossRef] [PubMed] [Google Scholar]
- Kümmerer K, Dionysiou DD, Olsson O, et al. A path to clean water. Science 2018; 361: 222-224. [Article] [CrossRef] [PubMed] [Google Scholar]
- Zhan S, Zhang H, Mi X, et al. Efficient fenton-like process for pollutant removal in electron-rich/poor reaction sites induced by surface oxygen vacancy over cobalt-zinc oxides. Environ Sci Technol 2020; 54: 8333-8343. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Hodges BC, Cates EL, Kim JH. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat Nanotech 2018; 13: 642-650. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Lyu L, Zhang L, He G, et al. Selective H2O2 conversion to hydroxyl radicals in the electron-rich area of hydroxylated C-g-C3N4/CuCo-Al2O3. J Mater Chem A 2017; 5: 7153-7164. [Article] [CrossRef] [Google Scholar]
- Lyu L, Zhang L, Hu C, et al. Enhanced Fenton-catalytic efficiency by highly accessible active sites on dandelion-like copper-aluminum-silica nanospheres for water purification. J Mater Chem A 2016; 4: 8610-8619. [Article] [Google Scholar]
- Deng K, Gu Y, Gao T, et al. Carbonized MOF-coated zero-valent Cu driving an efficient dual-reaction-center fenton-like water treatment process through utilizing pollutants and natural dissolved oxygen. ACS EST Water 2022; 2: 174-183. [Article] [CrossRef] [Google Scholar]
- Zhang X, Liang J, Sun Y, et al. Mesoporous reduction state cobalt species-doped silica nanospheres: An efficient Fenton-like catalyst for dual-pathway degradation of organic pollutants. J Colloid Interface Sci 2020; 576: 59-67. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Lyu L, Zhang L, Wang Q, et al. Enhanced Fenton catalytic efficiency of γ-Cu-Al2O3 by σ-Cu2+-ligand complexes from aromatic pollutant degradation. Environ Sci Technol 2015; 49: 8639-8647. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Lyu L, Zhang L, Hu C. Enhanced Fenton-like degradation of pharmaceuticals over framework copper species in copper-doped mesoporous silica microspheres. Chem Eng J 2015; 274: 298-306. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Li C, Cai X, Fang Q, et al. Peroxymonosulfate as inducer driving interfacial electron donation of pollutants over oxygen-rich carbon-nitrogen graphene-like nanosheets for water treatment. J Colloid Interface Sci 2022; 622: 272-283. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhang H, Lyu L, Fang Q, et al. Cation-π structure inducing efficient peroxymonosulfate activation for pollutant degradation over atomically dispersed cobalt bonding graphene-like nanospheres. Appl Catal B-Environ 2021; 286: 119912. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Zhang H, Li C, Lyu L, et al. Surface oxygen vacancy inducing peroxymonosulfate activation through electron donation of pollutants over cobalt-zinc ferrite for water purification. Appl Catal B-Environ 2020; 270: 118874. [Article] [CrossRef] [Google Scholar]
- Jin XG, Wu CY, Tian XM, et al. A magnetic-void-porous MnFe2O4/carbon microspheres nano-catalyst for catalytic ozonation: Preparation, performance and mechanism. Environ Sci Ecotech 2021; 7: 100110 [NASA ADS] [Google Scholar]
- Song Z, Wang M, Wang Z, et al. Insights into heteroatom-doped graphene for catalytic ozonation: active centers, reactive oxygen species evolution, and catalytic mechanism. Environ Sci Technol 2019; 53: 5337-5348. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Wang Y, Duan X, Xie Y, et al. Nanocarbon-based catalytic ozonation for aqueous oxidation: Engineering defects for active sites and tunable reaction pathways. ACS Catal 2020; 10: 13383-13414. [Article] [CrossRef] [Google Scholar]
- Loeb SK, Alvarez PJJ, Brame JA, et al. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ Sci Technol 2019; 53: 2937–2947 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Guo S, Zhu X, Zhang H, et al. Improving photocatalytic water treatment through nanocrystal engineering: Mesoporous nanosheet-assembled 3D BiOCl hierarchical nanostructures that induce unprecedented large vacancies. Environ Sci Technol 2018; 52: 6872-6880. [Article] [CrossRef] [PubMed] [Google Scholar]
- Zhang D, Lee C, Javed H, et al. Easily recoverable, micrometer-sized TiO2 hierarchical spheres decorated with cyclodextrin for enhanced photocatalytic degradation of organic micropollutants. Environ Sci Technol 2018; 52: 12402-12411. [Article] [CrossRef] [PubMed] [Google Scholar]
- Jiang G, Lan M, Zhang Z, et al. Identification of active hydrogen species on palladium nanoparticles for an enhanced electrocatalytic hydrodechlorination of 2,4-dichlorophenol in water. Environ Sci Technol 2017; 51: 7599-7605. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Liu K, Yu JCC, Dong H, et al. Degradation and mineralization of carbamazepine using an electro-fenton reaction catalyzed by magnetite nanoparticles fixed on an electrocatalytic carbon fiber textile cathode. Environ Sci Technol 2018; 52: 12667-12674. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Lu C, Fang Q, Hu C, et al. Sustainable micro-activation of dissolved oxygen driving pollutant conversion on Mo-enhanced zinc sulfide surface in natural conditions. Fund Res 2021; 3: 422–429 [Google Scholar]
- Lyu L, Yan D, Yu G, et al. Efficient destruction of pollutants in water by a dual-reaction-center fenton-like process over carbon nitride compounds-complexed Cu(II)-CuAlO2. Environ Sci Technol 2018; 52: 4294-4304. [Article] [CrossRef] [PubMed] [Google Scholar]
- Lyu L, Zhang L, Hu C. Galvanic-like cells produced by negative charge nonuniformity of lattice oxygen on d-TiCuAl-SiO2 nanospheres for enhancement of Fenton-catalytic efficiency. Environ Sci-Nano 2016; 3: 1483-1492. [Article] [CrossRef] [Google Scholar]
- Lyu L, Lu C, Sun Y, et al. Low consumption Fenton-like water purification through pollutants as electron donors substituting H2O2 consumption via twofold cation-π over MoS2 cross-linking g-C3N4 hybrid. Appl Catal B-Environ 2023; 320: 121871. [Article] [CrossRef] [Google Scholar]
- Wang Y, Zhang P, Lyu L, et al. Preferential destruction of micropollutants in water through a self-purification process with dissolved organic carbon polar complexation. Environ Sci Technol 2022; 56: 10849-10856. [Article] [CrossRef] [PubMed] [Google Scholar]
- Lagutschenkov A, Sinha RK, Maitre P, et al. Structure and infrared spectrum of the Ag+-phenol ionic complex. J Phys Chem A 2010; 114: 11053-11059. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Gebbie MA, Wei W, Schrader AM, et al. Tuning underwater adhesion with cation-π interactions. Nat Chem 2017; 9: 473-479. [Article] [Google Scholar]
- Wang L, Yan D, Lyu L, et al. Notable light-free catalytic activity for pollutant destruction over flower-like BiOI microspheres by a dual-reaction-center Fenton-like process. J Colloid Interface Sci 2018; 527: 251-259. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Lyu L, Deng K, Liang J, et al. The interaction of surface electron distribution-polarized Fe/polyimide hybrid nanosheets with organic pollutants driving a sustainable Fenton-like process. Mater Adv 2020; 1: 1083-1091. [Article] [CrossRef] [Google Scholar]
- Wang Y, Lyu L, Wang D, et al. Cation-π induced surface cleavage of organic pollutants with •OH formation from H2O for water treatment. iScience 2021; 24: 102874. [Article] [CrossRef] [PubMed] [Google Scholar]
- Cao W, Han M, Lyu L, et al. Efficient Fenton-like process induced by fortified electron-rich O microcenter on the reduction state Cu-doped CNO polymer. ACS Appl Mater Interfaces 2019; 11: 16496-16505. [Article] [CrossRef] [PubMed] [Google Scholar]
- Lyu L, Yu G, Zhang L, et al. 4-phenoxyphenol-functionalized reduced graphene oxide nanosheets: A metal-free fenton-like catalyst for pollutant destruction. Environ Sci Technol 2018; 52: 747-756. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Liu S, Kuznetsov AM, Han W, et al. Removal of dimethylarsinic acid (DMA) in the Fe/C system: Roles of Fe(II) release, DMA/Fe(II) and DMA/Fe(III) complexation. Water Res 2022; 213: 118093. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhang L, Yue Q, Yang K, et al. Enhanced phosphorus and ciprofloxacin removal in a modified BAF system by configuring Fe-C micro electrolysis: Investigation on pollutants removal and degradation mechanisms. J Hazard Mater 2018; 342: 705-714. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Ou X, Liu T, Zhong W, et al. Enabling high energy lithium metal batteries via single-crystal Ni-rich cathode material co-doping strategy. Nat Commun 2022; 13: 2319. [Article] [CrossRef] [MathSciNet] [PubMed] [Google Scholar]
- Zou L, Li J, Liu Z, et al. Lattice doping regulated interfacial reactions in cathode for enhanced cycling stability. Nat Commun 2019; 10: 3447. [Article] [Google Scholar]
- Kong XK, Chen CL, Chen QW. Doped graphene for metal-free catalysis. Chem Soc Rev 2014; 43: 2841-2857. [Article] [CrossRef] [PubMed] [Google Scholar]
- Lyu L, Hu C. Heterogeneous Fenton catalytic water treatment technology and mechanism. Prog Chem 2017; 29: 981–999 [Google Scholar]
- Gao T, Lu C, Hu C, et al. H2O2 inducing dissolved oxygen activation and electron donation of pollutants over Fe-ZnS quantum dots through surface electron-poor/rich microregion construction for water treatment. J Hazard Mater 2021; 420: 126579. [Article] [CrossRef] [PubMed] [Google Scholar]
- Gao T, Wang H, Xu J, et al. Enhanced water purification efficiency induced by trace H2O2 on the electron distribution-polarized unequilibrium surface of CuS/ZnO nanosheets. ACS ES&T Water 2022; 3: 79–85 [Google Scholar]
- Sun Y, Yi R, Hu C, et al. Enhanced purification efficiency for pharmaceutical wastewater through a pollutant-mediated H2O2 activation pathway over CuZnS nano-aggregated particles. Environ Sci-Nano 2022; 9: 4317-4324. [Article] [CrossRef] [Google Scholar]
- Zhang H, Lyu L, Hu C, et al. Enhanced purification of kitchen-oil wastewater driven synergistically by surface microelectric fields and microorganisms. Environ Int 2023; 174: 107878. [Article] [CrossRef] [PubMed] [Google Scholar]
- Jin Y, Li F, Li T, et al. Enhanced internal electric field in S-doped BiOBr for intercalation, adsorption and degradation of ciprofloxacin by photoinitiation. Appl Catal B-Environ 2022; 302: 120824. [Article] [CrossRef] [Google Scholar]
- Li H, Shang J, Yang Z, et al. Oxygen vacancy associated surface fenton chemistry: Surface structure dependent hydroxyl radicals generation and substrate dependent reactivity. Environ Sci Technol 2017; 51: 5685-5694. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Jiao Y, Zheng Y, Jaroniec M, et al. Origin of the electrocatalytic oxygen reduction activity of graphene-based catalysts: A roadmap to achieve the best performance. J Am Chem Soc 2014; 136: 4394-4403. [Article] [CrossRef] [PubMed] [Google Scholar]
- Duan X, O′Donnell K, Sun H, et al. Sulfur and nitrogen Co-doped graphene for metal-free catalytic oxidation reactions. Small 2015; 11: 3036-3044. [Article] [CrossRef] [PubMed] [Google Scholar]
- Liu S, Wang M, Qian T, et al. Selenium-doped carbon nanosheets with strong electron cloud delocalization for nondeposition of metal oxides on air cathode of zinc-air battery. ACS Appl Mater Interfaces 2019; 11: 20056-20063. [Article] [Google Scholar]
- Gao Y, Chen Z, Zhu Y, et al. New insights into the generation of singlet oxygen in the metal-free peroxymonosulfate activation process: Important role of electron-deficient carbon atoms. Environ Sci Technol 2020; 54: 1232-1241. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Gao Y, Zhu Y, Lyu L, et al. Electronic structure modulation of graphitic carbon nitride by oxygen doping for enhanced catalytic degradation of organic pollutants through peroxymonosulfate activation. Environ Sci Technol 2018; 52: 14371-14380. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Ma JC, Dougherty DA. The cation-π interaction. Chem Rev 1997; 97: 1303–1324 [CrossRef] [PubMed] [Google Scholar]
- Meot-Ner M, Deakyne CA. Unconventional ionic hydrogen bonds. 2. NH+.cntdot.cntdot.cntdot.pi. Complexes of onium ions with olefins and benzene derivatives. J Am Chem Soc 1985; 107: 474-479. [Article] [CrossRef] [Google Scholar]
- Zhu Y, Tang M, Zhang H, et al. Water and the cation-π interaction. J Am Chem Soc 2021; 143: 12397-12403. [Article] [CrossRef] [PubMed] [Google Scholar]
- Deng G, Pan S, Wang G, et al. Side-on bonded beryllium dinitrogen complexes. Angew Chem Int Ed 2020; 59: 10603-10609. [Article] [CrossRef] [PubMed] [Google Scholar]
- Han M, Lyu L, Huang Y, et al. In situ generation and efficient activation of H2O2 for pollutant degradation over CoMoS2 nanosphere-embedded rGO nanosheets and its interfacial reaction mechanism. J Colloid Interface Sci 2019; 543: 214-224. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Lyu L, Han M, Cao W, et al. Efficient Fenton-like process for organic pollutant degradation on Cu-doped mesoporous polyimide nanocomposites. Environ Sci-Nano 2019; 6: 798-808. [Article] [CrossRef] [Google Scholar]
- Deng K, Gao T, Fang Q, et al. Vanadium tetrasulfide cross-linking graphene-like carbon driving a sustainable electron supply chain from pollutants through the activation of dissolved oxygen and hydrogen peroxide. Environ Sci-Nano 2021; 8: 86-96. [Article] [CrossRef] [Google Scholar]
- Wang Y, Zhang P, Li T, et al. Enhanced Fenton-like efficiency by the synergistic effect of oxygen vacancies and organics adsorption on FexOy-d-g-C3N4 with Fe-N complexation. J Hazard Mater 2021; 408: 124818. [Article] [CrossRef] [PubMed] [Google Scholar]
- Jiang N, Lyu L, Yu G, et al. A dual-reaction-center Fenton-like process on –C≡N–Cu linkage between copper oxides and defect-containing g-C3N4 for efficient removal of organic pollutants. J Mater Chem A 2018; 6: 17819-17828. [Article] [CrossRef] [Google Scholar]
- Li L, Hu C, Zhang L, et al. Framework Cu-doped boron nitride nanobelts with enhanced internal electric field for effective Fenton-like removal of organic pollutants. J Mater Chem A 2019; 7: 6946-6956. [Article] [Google Scholar]
- Liang X, Wang D, Zhao Z, et al. Engineering the low-coordinated single cobalt atom to boost persulfate activation for enhanced organic pollutant oxidation. Appl Catal B-Environ 2022; 303: 120877. [Article] [CrossRef] [Google Scholar]
- Sun Y, Hu C, Lyu L. New sustainable utilization approach of livestock manure: Conversion to dual-reaction-center Fenton-like catalyst for water purification. npj Clean Water 2022; 5: 53. [Article] [Google Scholar]
- Cao W, Wang Z, Zhang P, et al. Water self-purification with zero external consumption by livestock manure resource utilization. Environ Sci Technol 2023; 57: 2837-2845. [Article] [CrossRef] [PubMed] [Google Scholar]
- Shi Y, Xie Z, Hu C, et al. Resourcelized conversion of livestock manure to porous cage microsphere for eliminating emerging contaminants under peroxymonosulfate trigger. iScience 2023; 26: 106139. [Article] [CrossRef] [PubMed] [Google Scholar]
- Zhao Z, Tan H, Zhang P, et al. Turning the inert element zinc into an active single-atom catalyst for efficient fenton-like chemistry. Angew Chem Int Ed 2023; 62: e202219178. [Article] [CrossRef] [PubMed] [Google Scholar]
- Lyu L, Cao W, Yu G, et al. Enhanced polarization of electron-poor/rich micro-centers over nZVCu-Cu(II)-rGO for pollutant removal with H2O2. J Hazard Mater 2020; 383: 121182. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Liao W, Wang D, Wang Y, et al. Surface-confined destruction of pollutants with H2O2 assistance over Cu0@CuOx-N-graphitic carbon suspensions. J Phys Chem C 2022; 126: 1366-1375. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Cao W, Hu C, Lyu L. Efficient decomposition of organic pollutants over nZVI/FeOx/FeNy-anchored NC layers via a novel dual-reaction-centers-based wet air oxidation process under natural conditions. ACS EST Eng 2021; 1: 1333-1341. [Article] [CrossRef] [MathSciNet] [Google Scholar]
- Yang J, Zhen X, Wang B, et al. The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat Commun 2018; 9: 840. [Article] [Google Scholar]
- Li X, Li XM, Jiang Y, et al. Structure-guided development of YEATS domain inhibitors by targeting π-π-π stacking. Nat Chem Biol 2018; 14: 1140-1149. [Article] [CrossRef] [PubMed] [Google Scholar]
- Wang Y, Fang Q, Xie Z, et al. Enhanced Fenton-like process via interfacial electron donating of pollutants over in situ Cobalt-doped graphitic carbon nitride. J Colloid Interface Sci 2022; 608: 673-682. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Yu G, Lyu L, Zhang F, et al. Theoretical and experimental evidence for rGO-4-PP Nc as a metal-free Fenton-like catalyst by tuning the electron distribution. RSC Adv 2018; 8: 3312-3320. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhuang Y, Liu Q, Kong Y, et al. Enhanced antibiotic removal through a dual-reaction-center Fenton-like process in 3D graphene based hydrogels. Environ Sci-Nano 2019; 6: 388-398. [Article] [CrossRef] [Google Scholar]
- Zhuang Y, Wang X, Liu Q, et al. N-doped FeOOH/RGO hydrogels with a dual-reaction-center for enhanced catalytic removal of organic pollutants. Chem Eng J 2020; 379: 122310. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Kong Y, Zhuang Y, Shi B. Tetracycline removal by double-metal-crosslinked alginate/graphene hydrogels through an enhanced Fenton reaction. J Hazard Mater 2020; 382: 121060. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhuang Y, Shi BY. Polymer hydrogels with enhanced stability and heterogeneous Fenton activity in organic pollutant removal. J Environ Sci 2019; 85: 147–155 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhuang Y, Wang X, Zhang L, et al. Fe-Chelated polymer templated graphene aerogel with enhanced Fenton-like efficiency for water treatment. Environ Sci-Nano 2019; 6: 3232-3241. [Article] [CrossRef] [Google Scholar]
- Wang X, Zhuang Y, Zhang J, et al. Pollutant degradation behaviors in a heterogeneous Fenton system through Fe/S-doped aerogel. Sci Total Environ 2020; 714: 136436. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Li L, Hu C, Zhang L, et al. More octahedral Cu+ and surface acid sites in uniformly porous Cu-Al2O3 for enhanced Fenton catalytic performances. J Hazard Mater 2021; 406: 124739. [Article] [CrossRef] [PubMed] [Google Scholar]
- Zhuang Y, Wang X, Zhang L, et al. Confinement Fenton-like degradation of perfluorooctanoic acid by a three dimensional metal-free catalyst derived from waste. Appl Catal B-Environ 2020; 275: 119101. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Xie Z, Zhou J, Wang J, et al. Novel Fenton-like catalyst γ-Cu-Al2O3-Bi12O15Cl6 with electron-poor Cu centre and electron-rich Bi centre for enhancement of phenolic compounds degradation and H2O2 utilization: The synergistic effects of σ-Cu-ligand, dual-reaction centres and oxygen vacancies. Appl Catal B-Environ 2019; 253: 28-40. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Xu S, Zhu H, Cao W, et al. Cu-Al2O3-g-C3N4 and Cu-Al2O3-C-dots with dual-reaction centres for simultaneous enhancement of Fenton-like catalytic activity and selective H2O2 conversion to hydroxyl radicals. Appl Catal B-Environ 2018; 234: 223-233. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Gao P, Song Y, Hao M, et al. An effective and magnetic Fe2O3-ZrO2 catalyst for phenol degradation under neutral pH in the heterogeneous Fenton-like reaction. Separation Purification Tech 2018; 201: 238-243. [Article] [CrossRef] [Google Scholar]
- Gao P, Chen X, Hao M, et al. Oxygen vacancy enhancing the Fe2O3-CeO2 catalysts in Fenton-like reaction for the sulfamerazine degradation under O2 atmosphere. Chemosphere 2019; 228: 521-527. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Panjwani MK, Wang Q, Ma Y, et al. High degradation efficiency of sulfamethazine with the dual-reaction-center Fe-Mn-SiO2 Fenton-like nanocatalyst in a wide pH range. Environ Sci-Nano 2021; 8: 2204-2213. [Article] [CrossRef] [Google Scholar]
- Wen S, Niu Z, Zhang Z, et al. In-situ synthesis of 3D GA on titanium wire as a binder- free electrode for electro-Fenton removing of EDTA-Ni. J Hazard Mater 2018; 341: 128-137. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhou X, Xu D, Chen Y, et al. Enhanced degradation of triclosan in heterogeneous E-Fenton process with MOF-derived hierarchical Mn/Fe@PC modified cathode. Chem Eng J 2020; 384: 123324. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Zhou H, Dong H, Wang J, et al. Cobalt anchored on porous N, P, S-doping core-shell with generating/activating dual reaction sites in heterogeneous electro-Fenton process. Chem Eng J 2021; 406: 125990. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Li X, Xiao C, Ruan X, et al. Enrofloxacin degradation in a heterogeneous electro-Fenton system using a tri-metal-carbon nanofibers composite cathode. Chem Eng J 2022; 427: 130927. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Xia QX, Zhang DJ, Yao ZP, et al. Investigation of Cu heteroatoms and Cu clusters in Fe-Cu alloy and their special effect mechanisms on the Fenton-like catalytic activity and reusability. Appl Catal B: Environ 2022; 302: 120662 [Google Scholar]
- Zhang X, Yao Z, Wang J, et al. High-capacity NCNT-encapsulated metal NP catalysts on carbonised loofah with dual-reaction centres over C–M bond bridges for Fenton-like degradation of antibiotics. Appl Catal B-Environ 2022; 307: 121205. [Article] [CrossRef] [Google Scholar]
- Zhang X, Yao Z, Zhou Y, et al. Theoretical guidance for the construction of electron-rich reaction microcenters on C-O-Fe bridges for enhanced Fenton-like degradation of tetracycline hydrochloride. Chem Eng J 2021; 411: 128535. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Liang H, Liu R, Hu C, et al. Synergistic effect of dual sites on bimetal-organic frameworks for highly efficient peroxide activation. J Hazard Mater 2021; 406: 124692. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Ren Y, Yu J, Zhang J, et al. An in-situ strategy to analyze multi-effect catalysis in iron-copper bimetals catalyzed Fenton-like processes. Appl Catal B-Environ 2021; 299: 120697. [Article] [CrossRef] [Google Scholar]
- Zhang N, Xue C, Wang K, et al. Efficient oxidative degradation of fluconazole by a heterogeneous Fenton process with Cu-V bimetallic catalysts. Chem Eng J 2020; 380: 122516. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Gu Y, Gao T, Zhang F, et al. Surface sulfur vacancies enhanced electron transfer over Co-ZnS quantum dots for efficient degradation of plasticizer micropollutants by peroxymonosulfate activation. Chin Chem Lett 2021; 33: 3829-3834. [Article] [Google Scholar]
- Han M, Liang G, Zhou S, et al. Zero-added conversion of chicken manure into dual-reaction-center catalyst for pollutant degradation triggered by peroxymonosulfate. Separation Purification Tech 2023; 317: 123763. [Article] [CrossRef] [Google Scholar]
- Li F, Lu Z, Li T, et al. Origin of the excellent activity and selectivity of a single-atom copper catalyst with unsaturated Cu-N2 sites via peroxydisulfate activation: Cu(III) as a dominant oxidizing species. Environ Sci Technol 2022; 56: 8765-8775. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Cao W, Lyu L, Deng K, et al. L-Ascorbic acid oxygen-induced micro-electronic fields over metal-free polyimide for peroxymonosulfate activation to realize efficient multi-pathway destruction of contaminants. J Mater Chem A 2020; 8: 810-819. [Article] [CrossRef] [Google Scholar]
- Cao W, Luo Y, Cai X, et al. π-π conjugation driving peroxymonosulfate activation for pollutant elimination over metal-free graphitized polyimide surface. J Hazard Mater 2021; 412: 125191. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Wang L, Huang X, Han M, et al. Efficient inhibition of photogenerated electron-hole recombination through persulfate activation and dual-pathway degradation of micropollutants over iron molybdate. Appl Catal B-Environ 2019; 257: 117904. [Article] [CrossRef] [Google Scholar]
- Liu B, Guo W, Si Q, et al. Atomically dispersed cobalt on carbon nitride for peroxymonosulfate activation: Switchable catalysis enabled by light irradiation. Chem Eng J 2022; 446: 137277. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Si Q, Guo W, Liu B, et al. Spin-states-assistance peroxymonosulfate absorption via Mn doped catalyst with/without light for BPA oxidation: The negative contribution of electrons transfer by light. Chem Eng J 2022; 443: 136399. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Zong M, Song D, Zhang X, et al. Facet-dependent photodegradation of methylene blue by hematite nanoplates in visible light. Environ Sci Technol 2021; 55: 677-688. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Liras M, Barawi M, de la Peña O’Shea VA. Hybrid materials based on conjugated polymers and inorganic semiconductors as photocatalysts: from environmental to energy applications. Chem Soc Rev 2019; 48: 5454-5487. [Article] [CrossRef] [PubMed] [Google Scholar]
- Zhou Z, Zhang Y, Shen Y, et al. Molecular engineering of polymeric carbon nitride: advancing applications from photocatalysis to biosensing and more. Chem Soc Rev 2018; 47: 2298-2321. [Article] [CrossRef] [PubMed] [Google Scholar]
- Li F, Han M, Jin Y, et al. Internal electric field construction on dual oxygen group-doped carbon nitride for enhanced photodegradation of pollutants under visible light irradiation. Appl Catal B-Environ 2019; 256: 117705. [Article] [CrossRef] [Google Scholar]
- Li F, Li T, Zhang L, et al. Enhancing photocatalytic performance by direct photo-excited electron transfer from organic pollutants to low-polymerized graphitic carbon nitride with more C-NH/NH2 exposure. Appl Catal B-Environ 2021; 296: 120316. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Li F, Lu Z, Zhang P, et al. Boosting the extra generation of superoxide radicals on graphitic carbon nitride with carbon vacancies by the modification of pollutant adsorption for high-performance photocatalytic degradation. ACS EST Eng 2022; 2: 1296-1305. [Article] [CrossRef] [Google Scholar]
- Li J, Cai L, Shang J, et al. Giant enhancement of internal electric field boosting bulk charge separation for photocatalysis. Adv Mater 2016; 28: 4059-4064. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Yang C, Ma BC, Zhang L, et al. Molecular engineering of conjugated polybenzothiadiazoles for enhanced hydrogen production by photosynthesis. Angew Chem Int Ed 2016; 55: 9202-9206. [Article] [CrossRef] [PubMed] [Google Scholar]
- Li H, Tu W, Zhou Y, et al. Z-scheme photocatalytic systems for promoting photocatalytic performance: Recent progress and future challenges. Adv Sci 2016; 3: 1500389. [Article] [CrossRef] [Google Scholar]
- Fan X, Zhang L, Cheng R, et al. Construction of graphitic C3N4-based intramolecular donor-acceptor conjugated copolymers for photocatalytic hydrogen evolution. ACS Catal 2015; 5: 5008-5015. [Article] [Google Scholar]
- Chen Z, Li S, Peng Y, et al. Tailoring aromatic ring-terminated edges of g-C3N4 nanosheets for efficient photocatalytic hydrogen evolution with simultaneous antibiotic removal. Catal Sci Technol 2020; 10: 5470-5479. [Article] [CrossRef] [Google Scholar]
- Chu S, Wang Y, Guo Y, et al. Band structure engineering of carbon nitride: In search of a polymer photocatalyst with high photooxidation property. ACS Catal 2013; 3: 912-919. [Article] [CrossRef] [Google Scholar]
- Guo Y, Zhou Q, Nan J, et al. Perylenetetracarboxylic acid nanosheets with internal electric fields and anisotropic charge migration for photocatalytic hydrogen evolution. Nat Commun 2022; 13: 2067. [Article] [Google Scholar]
- Wang Y, Zhang P, Lyu L, et al. Efficient destruction of humic acid with a self-purification process in an Fe0-FeyCz/Fex-GZIF-8-rGO aqueous suspension. Chem Eng J 2022; 446: 136625. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Nie J, Zou J, Yan S, et al. Photosensitized transformation of peroxymonosulfate in dissolved organic matter solutions under simulated solar irradiation. Environ Sci Technol 2022; 56: 1963-1972. [Article] [CrossRef] [PubMed] [Google Scholar]
- Gu H, Liu X, Liu X, et al. Adjacent single-atom irons boosting molecular oxygen activation on MnO2. Nat Commun 2021; 12: 5422. [Article] [Google Scholar]
- Gong X, Yang Z, Peng L, et al. In-situ synthesis of hydrogen peroxide in a novel Zn-CNTs-O2 system. J Power Sources 2018; 378: 190-197. [Article] [NASA ADS] [CrossRef] [Google Scholar]
- Lyu J, Niu L, Shen F, et al. In situ hydrogen peroxide production for selective oxidation of benzyl alcohol over a pd@hierarchical titanium silicalite catalyst. ACS Omega 2020; 5: 16865-16874. [Article] [CrossRef] [PubMed] [Google Scholar]
- Li R, Huang Y, Zhu D, et al. Improved oxygen activation over a carbon/Co3O4 nanocomposite for efficient catalytic oxidation of formaldehyde at room temperature. Environ Sci Technol 2021; 55: 4054-4063. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
All Figures
 |
Figure 1 Common interfacial reaction mechanism of the MEF-based technologies. |
| In the text | |
 |
Figure 2 Molecular orbitals obtained from linear combination of atomic orbitals (LCAO) in molecules. |
| In the text | |
 |
Figure 3 Schematic digram of electron cloud density distribution on d-TiCuAl-SiO2 Ns. Adapted with permission from ref. [42], Copyright©2017, Progress in Chemistry. |
| In the text | |
 |
Figure 4 (A) TEM image and FESEM of DCAS Ns. (B) Schematic illustration of pollutants or H2O2 in contact with the catalyst with the active component in the bulk, the multiparous catalyst and DCAS Ns. (C) XPS spectra for Cu 2p3/2 for DCS Ns and Cu 2p3/2 for DCAS Ns. (D) EXAFS curve fitting of DCS Ns and DCAS Ns (solid line-experimental signals, dotted line-fitted curves). (E) BPA degradation and TOC removal in different suspensions with H2O2. (F) H2O2 decomposition and the utilization efficiency of H2O2 during the degradation of BPA in the DCAS Ns suspension. (G) BMPO spin trapping ESR spectra recorded at ambient temperature for various DCAS Ns samples with H2O2 in aqueous dispersion for BMPO–•OH and in methanol dispersion for BMPO–HO2•/O2•−: (1) fresh DCAS Ns; (2) DCAS Ns adsorbed BPA; and (3) DCAS Ns used for 15 min in the Fenton reaction with BPA. Adapted with permission from ref. [9], Copyright©2016, The Royal Society of Chemistry. |
| In the text | |
 |
Figure 5 (A) Fourier transforms of k3-weighted EXAFS oscillations obtained at the Cu K-edge of the various samples. (B) Solid EPR spectra of various samples. (C) Cu 2p3/2 XPS spectra for d-TiCuAl-SiO2 Ns, d-Cu-SiO2, Ns d-CuAl-SiO2 Ns and Ti 2p XPS spectra for d-TiCuAl-SiO2 Ns. The inset shows the LMM X-ray induced Auger parameter for the sample. (D) CV of various electrodes prepared from the corresponding samples. (E) Fenton catalytic degradation of BPA in various suspensions at initial pH 7. (F) The utilization efficiency of H2O2 in the d-Cu-SiO2 Ns, d-CuAl-SiO2 Ns and d-TiCuAl-SiO2 Ns suspensions. The inset shows the corresponding decomposition of H2O2. Adapted with permission from ref. [27], Copyright©2016, The Royal Society of Chemistry. |
| In the text | |
 |
Figure 6 (A) Fourier transforms spectra of Zn K-edge EXAFS for OV-CoZnO MPs and ZnO. (B) Raman spectra of the OV-CoZnO MPs and ZnO samples. (C) EPR spectra of the OV-CoZnO MPs, OV(L)-CoZnO MPs and ZnO solid samples. (D) RhB degradation curves in various suspensions with H2O2 (the inset shows the pseudo-first-order kinetic rate plots of ZnO and OV-CoZnO MPs Fenton-like systems). (E) Optimized adsorption/reaction model for H2O2 and typical hydroxylated pollutant fragment (phenol) on the surface of OV-CoZnO MPs through DFT calculations. DFT total energy (EDFT) and adsorption energy (ΔE) for H2O2 and phenol at different sites on the surface of OV-CoZnO MPs. Adapted with permission from ref. [6], Copyright©2020, American Chemical Society. |
| In the text | |
 |
Figure 7 (A) Fourier transforms of k3-weighted EXAFS oscillations obtained at the Cu K-edge. (B) XPS spectra in Cu 2p3/2 for CuCo-Al2O3 and OH-CCN/CuCo-Al2O3. (C) EPR spectra of all of the fresh solid samples. (D) Degradation of BPA in various suspensions with H2O2 and TOC removal during the degradation of BPA in various suspensions. (E) The structure and two-dimensional valence-electron density color-filled maps of carbon-doped g-C3N4 growing on the surface of Cu-doped g-Al2O3 through the connection of C-O-Cu: the valence-electron density map in the Cu-O1-C1 plane, O1-C1-C2 plane and O1-C1-N1 plane. (F) BMPO spin-trapping EPR spectra for HO2•/O2•− and •OH in various methanol dispersions without H2O2. Adapted with permission from ref. [8], Copyright©2017, The Royal Society of Chemistry. |
| In the text | |
 |
Figure 8 (A) XPS spectra in Cu 2p and O 1s for CN-Cu(II)-CuAlO2. (B) EPR spectra of the fresh g-C3N4, CuAlO2, and CN-Cu(II)-CuAlO2 samples. (C) DFT calculations for the optimized structure (left) and the corresponding two-dimensional valence-electron density color-filled maps (right) of the CN-Cu(II)-CuAlO2 model in -Cu(II)-CN vision fragment and -CN vision fragment. (D) BPA degradation curves in various suspensions with H2O2 (the inset shows the corresponding kinetic curves) and TOC removal curves during BPA degradation. (E) H2O2 decomposition curves during BPA degradation in CuAlO2 and CN-Cu(II)-CuAlO2 suspensions. (F) EPR spectra for •OH and (d) HO2•/O2•− in various suspensions in the presence of H2O2 with and without pollutants. Adapted with permission from ref. [26], Copyright©2018, American Chemical Society. |
| In the text | |
 |
Figure 9 (A) Fourier transforms of Cu K-edge EXAFS for Cu foil, Cu2O, and RSC-CNOP. (B) Solid-state EPR of various samples. (C) The valence-electron density maps of the RSC-CNOP model fragment in the Cu-O-C calculated-plane, O-C-C(N) calculated-plane, and aromatic ring calculated-plane and ESP map of the RSC-CNOP model fragment. (D) Conversion curves of BPA in different Fenton-like suspensions (inset shows the corresponding TOC removal curves). (E) DMPO spin-trapping EPR spectra for HO2•/O2•− and •OH in the absence of H2O2, HO2•/O2•− and •OH in the presence of H2O2 in RSC-CNOP suspensions with/without pollutants. Adapted with permission from ref. [35], Copyright©2019, American Chemical Society. |
| In the text | |
 |
Figure 10 (A) Fourier transforms of k3-weighted EXAFS oscillations obtained at the Cu K-edge of Cu foil, Cu2O, CuO and nZVC-Cu(II)-rGO. (B) Cu 2p and C 1s of nZVC-Cu(II)-rGO. (C) Formation of active electron-poor/rich micro-regions on nZVC-Cu(II)-rGO surface. (D) 2-CP degradation curves in various suspensions with H2O2. (E) Actual and stoichiometric H2O2 consumptions and the utilization efficiency of H2O2 during 2-CP degradation in the nZVC-Cu(II)-rGO (5 wt %) suspension. (F) BMPO spin-trapping EPR spectra for HO2•/O2•− in the nZVC-Cu(II)-rGO suspensions in the absence of H2O2 with/without pollutants. Adapted with permission from ref. [69], Copyright©2019, Elsevier. |
| In the text | |
 |
Figure 11 (A) Schematic illustration for the synthesis routes of CMS-rGO NSs. (B) Fourier transforms of k3-weighted EXAFS oscillations obtained at the Mo K-edge of MS, MS-rGO NSs and CMS-rGO NSs and EXAFS curve fittings of CMS-rGO NSs on [χ(R)] and Re[χR)]. (C) Decomposition curves of different dye pollutants in CMS-rGO NSs suspensions with H2O2 and kinetic curves for RhB degradation in various suspensions with H2O2. (D) BMPO spin-trapping EPR spectra for HO2•/O2•− and •OH in the CMS-rGO NSs suspensions in the absence/presence of H2O2 with/without dye pollutants. (E) Schematic for the novel Fenton-like reaction process in CMS-rGO NSs systems under Catalyst+H2O/O2+H2O2+Dye conditions. Adapted with permission from ref. [58], Copyright©2019, Elsevier. |
| In the text | |
 |
Figure 12 (A) C 1s XPS spectra of POP-rGO NSs and EPR spectra of GO, rGO, POP-rGO NSs, and POP. (B) Distributions of electrostatic potential (ESP) for different graphene fragments that O atom located in different positions via DFT calculations: No O atom (represents pure graphene), O atom in the surface oxygen functional groups (represents GO or rGO (with –OH groups)), and O atom in C–O–C bonding bridge that connected the graphene and POP molecule (represents POP-rGO NSs fragment). (C) 2-CP degradation curves in various suspensions with H2O2 and actual and stoichiometric H2O2 consumptions and the utilization efficiency of H2O2 during 2-CP degradation in the POP-rGO NSs suspension. Adapted with permission from ref. [36], Copyright©2017, American Chemical Society. |
| In the text | |
 |
Figure 13 (A) Structural characterization of CuSA-NC catalyst. (B) Optimized atomic Cu-N4 and Cu-N2 configuration, partial density of states (PDOS) for Cu-N4 and Cu-N2 sites, and the reaction pathway of PDS activation and 2,4-DCP oxidation on CuSA-NC. (C) ECs degradation performance in different systems. (D) 2,4-DCP concentration in the effluent from a continuous-flow reactor consisting of the CuSA-NC-filled column (inset: diagram of the continuous-flow reactor). Adapted with permission from ref. [101], Copyright©2022, American Chemical Society. |
| In the text | |
 |
Figure 14 (A) SEM and TEM images of OLAA-CN NSs. (B) Characterization of OLAA-CN NSs. (C) Decomposition curves of different ECs in OLAA-CN NSs/PMS suspensions. (D) Interface reaction mechanism of OLAA-CN NSs process for pollutant removal under initiation of PMS. Adapted with permission from ref. [14], Copyright©2022, Elsevier. |
| In the text | |
 |
Figure 15 (A) Schematic illustration of preparation for ACN*. (B) The two-dimensional valence-electron density color-filled maps of CN, ACN, and ACN* (multi-directional spliced diagram). (C) BPA degradation experiments. (D) Transient photocurrent responses of ACN* adsorbed different pollutants. (E) BMPO spin-trapping EPR spectra for O2•−. (F) Electrostatic potential (ESP) maps of the g-C3N4 model compounds, i.e., monomer (leftmost column), dimer (second column), trimer (third column) and tetramer (rightmost column). (G) Solid EPR spectra of BCN and LCN before and after adsorbing different pollutants. (H) UV-vis DRS spectra of BPA, BCN and LCN under different condition. (I) Transient photocurrent response spectra of LCN before and after adsorbing different pollutants in air/Ar condition. (J) BMPO spin-trapping EPR spectra for BMPO-O2•− in the HTCN system. (A)–(E) Adapted with permission from ref. [110], Copyright©2019, Elsevier; (F)–(I) adapted with permission from ref. [111], Copyright©2021, Elsevier; (J) adapted with permission from ref. [112], Copyright©2022, American Chemical Society. |
| In the text | |
 |
Figure 16 (A) Schematic illustration of the separation and migration of electrons and holes in the bulk of the pure and C-doped Bi3O4Cl. (B) Charge distribution of S-BiOBr. (C) Removal efficiency of CIP by S-BiOBr-3 in the dark after 30, 60, and 120 s of illumination. (D) EPR spectra for BMPO spin trapping of O2•− and R• in S-BiOBr with/without CIP adsorption. (A) Adapted with permission from ref. [113], Copyright©2016, Wiley-VCH; (B)–(D) adapted with permission from ref. [47], Copyright©2022, Elsevier. |
| In the text | |
 |
Figure 17 (A) XRD patterns of ZnS and MZS-1. (B) Raman spectra of ZnS, MoS2 and MZS-1. (C) Local potential of MZS-1 by DFT calculation. (D) Stability test of MZS-1 reactor in degrading RhB. (E) EPR spectra for BMPO-•OH, BMPO-O2•− and TEMPO-1O2 in various suspensions. (F) Schematic illustration of sustainable microactivation of DO and ECs conversion on the MZS-1 surface. Adapted with permission from ref. [25], licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
| In the text | |
 |
Figure 18 (A) Schematic illustration of the preparation of Fe0-FeyCz/Fex-GZIF-8-rGO. (B) ESP distribution for different structural model fragments of Fe0-FeyCz/Fex-GZIF-8-rGO. (C) TOC removal of various ECs. (D) BPA degradation in the aqueous suspension of Fe0-FeyCz/Fex-GZIF-8-rGO under different reaction conditions specified in the panel. (E) BMPO spin-trapping EPR spectra for O2•− in air-saturated methanol suspensions with/without BPA. (F) BMPO spin-trapping EPR spectra for •OH in the air-saturated D2O suspensions with/without BPA. Adapted with permission from ref. [34], licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Licence. |
| In the text | |
 |
Figure 19 Degradation curves of various ECs (A) without HA and (B) with HA in ultrapure water. Degradation curves of BPA, DP and ATZ in (C) municipal wastewater and (D) raw drinking water suspension. (E) Highest occupied molecular orbital (HOMO) for DP, BPA and ATZ on the HA model fragments with isovalue of 0.015 bohr−3/2. (F) Solid EPR spectra and chronoamperometry curves of various samples. BMPO spin-trapping EPR spectra for (G) O2•− and (H) •OH with/without DP in the Fe0-FeyCz/Fex-GZIF-8-rGO/HA air-saturated aqueous suspensions. Reaction conditions: (A), (B) catalyst concentration 0.6 g/L, initial ECs concentration 3.0 mg/L, initial HA concentration 10.0 mg/L, temperature 35°C; (C), (D) catalyst concentration 0.6 g/L, initial ECs concentration 3.0 mg/L, temperature 35°C. Adapted with permission from ref. [29], Copyright©2022, American Chemical Society. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

