| Issue |
Natl Sci Open
Volume 4, Number 4, 2025
|
|
|---|---|---|
| Article Number | 20250022 | |
| Number of page(s) | 11 | |
| Section | Materials Science | |
| DOI | https://doi.org/10.1360/nso/20250022 | |
| Published online | 09 July 2025 | |
RESEARCH ARTICLE
Cryo-synthesized Prussian blue analogues as advanced cathode materials for potassium-ion batteries
1
School of Physics and Electronics, Hunan University, Changsha 410082, China
2
Greater Bay Area Institute for Innovation, Hunan University, Guangzhou 511300, China
3
College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China
4
Department of Physics and Astronomy, Clemson Nanomaterials Institute, Clemson University, Clemson, SC 29634, USA
5
State Key Laboratory of Advanced Design and Manufacturing for Vehicle Body, Hunan University, Changsha 410082, China
* Corresponding authors (emails: ctgao@hnu.edu.cn (Caitian Gao); luba2012@hnu.edu.cn (Bingan Lu))
Received:
9
June
2025
Revised:
24
June
2025
Accepted:
24
June
2025
Prussian blue analogues (PBAs) are increasingly recognized as promising cathode materials for potassium-ion batteries (PIBs) owing to their low cost, distinctive open structure, and high theoretical capacity. However, PBAs still face challenges related to low crystallinity and elevated crystal water content, adversely affecting their performance as cathodes. This study proposes a straightforward low-temperature synthesis method for PBAs termed cryo-synthesized PBAs. The nucleation and growth processes were effectively slowed down by precisely controlling the synthesis temperature. This approach successfully yields PBAs with enhanced crystallinity and uniform particle size. The PBA electrode utilized as the cathode in PIBs demonstrates remarkable long-term cycling stability over 10,000 cycles at a current density of 2000 mA g−1. We believe these findings will promote the widespread adoption of PBAs in aqueous PIBs, paving the way for large-scale, cost-effective applications.
Key words: Prussian blue analogues / cryo-synthesized PBAs / cathode materials / aqueous potassium-ion batteries / long-term cycling stability
© The Author(s) 2025. Published by Science Press and EDP Sciences.
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In recent decades, potassium-ion batteries (PIBs) have garnered increasing attention due to potassium’s abundant resources and potentially low cost compared to lithium [1–10]. Although PIBs exhibit a theoretical energy density comparable to lithium-ion batteries (LIBs), the practical development and implementation of high-performance cathode materials remain a critical challenge. Specifically, traditional cathode materials for PIBs often suffer from structural instability during potassium insertion/extraction processes, leading to rapid capacity fading and poor cycling stability [11–16]. Thus, the cathode materials for PIBs are key factors affecting the battery’s overall performance, including energy density and cycling stability.
The PIBs’ cathode materials primarily include layered materials, polyanion compounds, Prussian blue and its analogs (PB/PBAs), as well as organic materials [17–20]. Among these, PB/PBAs have attracted significant attention due to their excellent electrochemical performance and good cycling stability. PB/PBAs possess a unique framework structure that allows these materials to accommodate potassium ions effectively and provide rapid ionic conductivity [21–23]. Exploring new synthesis methods is crucial in developing high-performance cathode materials for PIBs. Coprecipitation is a common method for PB/PBAs, which is relatively straightforward and cost-effective. However, the fast coprecipitation process often results in aggregates of particles with the incorporation of numerous Fe(CN)6 vacancies, interstitial water, and crystallized water [24–26]. Therefore, regulating the reaction rate of coprecipitation has become the focus of research. Recently, controlling the release rate of transition metal ions in the reaction solution has been the main strategy. Deng et al. [27] incorporated the EDTA-2K (ethylenediamine-N,N,N',N'-tetraacetic acid dipotassium) salt into the reaction solution to form a stable chelate, Mn[(EDTA)]2− with Mn2+, thereby reducing the reaction rate between Mn2+ and [Fe(CN)6]4− during the coprecipitation process and enhancing the crystallization of the product. The obtained MnHCF achieves excellent potassium storage performance. In addition, citrate salts, including sodium citrate and potassium citrate, are extensively employed to form complexes with transition metal ions. This not only helps modulate the reaction rate of coprecipitation but also renders the reaction process more gentle and controllable [28]. Indeed, crystal nucleation and growth processes are intricately related to temperature; thus, adjusting the synthesis temperature can modulate the nucleation and growth rates. Currently, most studies focus on elevating the ambient temperature during material synthesis. For instance, Liu et al. [29] synthesized iron-based PBAs by coprecipitation at 5°C to 60°C. They observed that at 60°C, PBAs undergo a phase transition from cubic to a monoclinic phase, exhibiting increased charging and discharging specific capacity. Kim et al. [30] synthesized cobalt hexacyanoferrate (CoHCF) by coprecipitation at temperatures ranging from 5°C to 70°C and found that higher temperatures led to larger particles with lower Fe vacancies and higher Na content. In contrast, lower temperatures (5°C) produced smaller particles with higher Fe vacancies, resulting in superior rate capability and cyclability, albeit with slightly reduced capacity. However, these studies overlooked the parabolic relationship between crystal nucleation growth rate and temperature (Figure 1A) [31,32]. Consequently, based on the correlation between temperature and crystal nucleation growth rate, it can be inferred that an appropriate reduction in temperature can decelerate the nucleation and growth rates of PBAs.
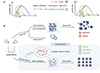 |
Figure 1 Schematic illustration of nucleation-growth mechanisms in Prussian blue analogues (PBAs) synthesis and their temperature-dependent crystallization control. (A) PBA’s nucleation and growth kinetics are largely governed by temperature, where Tf and Tm1/Tm2 represent the solidification temperature (addition EG) and the fastest rate of nucleation/growth temperature of the precursor solution. (B) The scenarios of PBA synthesis under different temperatures. Conventional synthesis routes exhibit limited control over particle size distribution due to rapid nucleation under ambient conditions. Thermodynamically favored growth leads to particle aggregation with heterogeneous size distribution at room temperature (upper panel). The sub-zero synthesis cannot be achieved due to the freezing of the aqueous precursor solution (middle panel). Our developed cryo-synthesis approach introduces hydrogen-bond disrupting additives, achieving monodisperse nanocrystals with enhanced crystallinity (lower panel). |
In this work, we proposed a cryo-synthesis strategy, as shown in Figure 1B. Typically, when the temperature is reduced to below 0°C, ice formation occurs within the precursor solutions, thereby disrupting the material synthesis process. To address this issue, we introduced ethylene glycol (EG), an antifreeze agent, to lower the freezing point of water [33,34]. Additionally, owing to the interaction between EG molecules and H2O molecules, the presence of EG helps prevent a portion of the water from freezing inside the PBA’s crystal structure during synthesis, reducing the amount of crystallized water and interstitial water within the PBA. By employing this strategy, we successfully synthesized a MnFe PBA with a more uniform particle size distribution (Figure 1B bottom), designated as MnFe_PBA_Cryo_−10°C. Consequently, our results show that PIBs with MnFe_PBA_Cryo_−10°C exhibit a high-rate performance and achieve remarkable long-term cycling stability of 10000 cycles at 2000 mA g−1. Near-unity capacity retention is achieved with a high initial capacity of 103 mAh g−1 at 500 mA g−1 over 3500 cycles in the half cell. The MnFe_PBA_Cryo_−10°C || PTCDI full cell also exhibits an excellent performance with capacity retention of 80.3%, and the average Coulombic efficiency approaches 100% over 1500 cycles, indicating promising potential for practical applications of PIBs.
RESULTS AND DISCUSSION
Firstly, we applied coprecipitation methods and improved coprecipitation methods to synthesize three types of MnFe_PBA, named MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG, and MnFe_PBA_Cryo_−10°C, with the synthesis method detailed in the experimental section. Figure 2A–C show the refined X-ray diffraction (XRD) patterns of the three samples and crystal structures of the MnFe_PBA_Cryo_−10°C (inset in Figure 2C). Figure 2C shows that the peaks of the MnFe_PBA_Cryo_−10°C are relatively sharper, indicating that this sample has the best crystallinity among the three samples. The corresponding crystal structure of MnFe_PBA_Cryo_−10°C is such that Mn coordinates with the N in C≡N−, while Fe coordinates with the C in C≡N−, forming an open three-dimensional framework that allows for fast ionic transport through large channels (inset in Figure 2C). We employed thermogravimetric analysis (TGA) and inductively coupled plasma-optical emission spectroscopy (ICP-OES) to determine the chemical formula of the three samples, which are K1.78Mn[Fe(CN)6]0.9·1.15H2O, K1.77Mn[Fe(CN)6]0.92·1.01H2O, and K1.69Mn[Fe(CN)6]0.89·0.87H2O for MnFe_PBA-RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C, respectively. These chemical formulas indicate that the defects and water content decrease sequentially from MnFe_PBA_RT w/o EG to MnFe_PBA_RT w EG and then MnFe_PBA_Cryo_−10°C, highlighting EG’s role in lowering the synthesis temperature during cryo-synthesis. The scanning electron microscopy (SEM) images indicate that all three samples comprise nanoparticles (insets of Figure 2D and E). We used the Nano Measurer Software to determine the particle size distribution of the three samples, as shown in the bar charts in Figure 2D and E. Next, Gaussian fitting was used to fit the particle distribution data, which indicated that the MnFe_PBA_Cryo_−10°C particle is the most uniform. This uniformity arises from the lower synthesis temperature, which allows for slower crystal nucleation and growth, resulting in relatively consistent particle sizes. Figure 2G is a transmission electron microscopy (TEM) image of the MnFe_PBA_Cryo_−10°C, which also shows that the particles of the MnFe_PBA_Cryo_−10°C have good crystallinity. TEM-energy dispersive spectroscopy (EDS) mapping study detected elemental Mn, Fe, and K in the MnFe_PBA_Cryo_−10°C (shown in Supplementary information). It confirms the even distribution of K, Fe, and Mn, further indicating the MnFe_PBA_Cryo_−10°C’s crystal structure. X-ray photoelectron spectroscopy (XPS) survey spectra also confirm the existence of the K, Mn, Fe, C, and N elements. The full spectrum is displayed in Supplementary information in Figure S3. In the high-resolution Mn 2p spectra (Figure 2H), peaks at 653 eV/641.4 eV and 654.5 eV/643.05 eV predominantly represent Mn2+ and Mn3+, respectively. In Fe 2p spectra (Figure 2I), peaks at 721.59 eV/708.67 eV and 724.01 eV/709.82 eV predominantly represent Fe2+ and Fe3+, respectively [35–38].
 |
Figure 2 Structural and compositional characterization of MnFe_PBA synthesized under ambient and cryogenic conditions. (A–F) The XRD patterns and the Rietveld refinement of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C. The SEM images and the statistics of the particle size distribution of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C, respectively. (G) Transmission electron microscopy (TEM) image of MnFe_PBA_cryo_−10°C. The XPS fine scan of Mn 2p (H) and Fe 2p (I) spectra of MnFe_PBA_cryo_−10°C. |
The electrochemical performance comparison of MnFe_PBA_RT w/o EG, MnF_PBA_RT w EG, and MnFe_PBA_Cryo_−10°C is shown in Figure 3. Figure 3A displays the cyclic voltammetry (CV) curves of the three samples at a scan rate of 0.1 mV s−1 during the second cycle. A progressive decrease in voltage polarization is observed from MnFe_PBA_RT_w/o_EG to MnFe_PBA_Cryo_−10°C, suggesting improved reaction kinetics. These changes may relate to the materials’ uniform particle structure and ionic diffusion properties, thereby affecting their overall electrochemical characteristics. Figure 3B compares the cycling performance of these three samples used as cathode materials in a three-electrode system for aqueous potassium-ion batteries at a current density of 500 mA g−1. As shown in Figure 3B, although the material synthesized via the conventional coprecipitation method has a relatively high initial discharge capacity, its capacity rapidly decreases with increasing cycling numbers. In contrast, the MnFe_PBA_RT w EG sample shows improved cycling performance. Impressively, the MnFe_PBA_Cryo_−10°C sample not only surpasses the other two samples in initial coulombic efficiency (85% ICE) but also retains 88% of its capacity after 3500 cycles, demonstrating excellent cycling stability.
 |
Figure 3 The electrochemical performances of MnFe_PBA synthesized at ambient and −10°C. (A) Cyclic voltammetry (CV) curves of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG, and MnFe_PBA_Cryo_−10°C with their respective polarization voltages. (B) Cycling performances of MnFe_PBA at room temperature and cryo-synthesis at the current densities of 500 mA g−1. (C) Rate performance of MnFe_PBA_Cryo_−10°C at current densities from 500 to 1500 mA g−1. (D) Charge-discharge curves of MnFe_PBA_Cryo_−10°C at current densities from 500 to 1500 mA g−1. (E) Long-term cycling performances of MnFe_PBA_Cryo_−10°C at the current densities of 2000 mA g−1. (F) Comparison of the capacity retention in this work with previously reported PBAs. |
To verify the rate performance of the MnFe_PBA_Cryo_−10°C, we conducted charge-discharge tests at current densities from 500 to 1500 mA g−1, and the results are shown in Figure 3C and D. MnFe_PBA_Cryo_−10°C exhibits reversible discharge capacities of 120, 108, 100, 97, and 93 mAh g−1 at 500, 800, 1000, 1200, and 1500 mA g−1, respectively. Notably, when the current density increased from 500 to 1500 mA g−1, the capacity retention was 77%, indicating that the crystal structure remains relatively stable during rapid ionic transport. Figure 3E illustrates the cycling performance of MnFe_PBA_Cryo_−10°C at a current density of 2000 mA g−1, where the battery can still charge and discharge effectively after 10,000 cycles, further highlighting the excellent cycling stability of MnFe_PBA_Cryo_−10°C even at higher current densities. Furthermore, it is also noteworthy that compared to previous battery studies, the electrode materials synthesized using the sub-zero temperature method possess higher capacities and outstanding capacity retention, as shown in Figure 3F [26,39–56].
We hypothesize that the particles of this material are relatively uniform, which makes the diffusion of the electrolyte more rapid, as shown in Figure 4A. COMSOL simulations and complementary electrochemical characterization techniques were employed to validate this hypothesis. As shown in Figure 4B, we first obtained two data sets with non-uniform and uniform particle distributions using Gaussian distribution. Then, we simulated the diffusion process of the electrolyte from 0 to 2 ms in these two samples, respectively. We adopt the method of using concentration difference to describe the diffusion rate. Figure 4B shows that at the same time, the electrolyte diffuses faster in the MnFe_PBA_Cryo_−10°C because of its uniform particles. At 1 ms, the electrolyte concentration in PBA with non-uniform particles is approximately distributed between 0.5 and 1 mol m−3, while that with uniform particles is approximately distributed between 0.8 and 1 mol m−3. Furthermore, we analyzed the diffusion coefficient of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C using the galvanostatic intermittent titration technique (GITT), as shown in Figure 4C. Under the same test conditions, the diffusion coefficient of the MnFe_PBA_Cryo_−10°C material is slightly higher than that of MnFe_PBA_RT w/o EG and MnFe_PBA_RT w EG. Additionally, cells using MnFe_PBA_RT w/o EG and MnFe_PBA_RT w EG exhibited charging issues, particularly in MnFe_PBA_RT w/o EG, which showed the most severe charging difficulty. Finally, we also measured the electrochemical impedance spectroscopy (EIS) of the material and adopted the equivalent circuit and distribution of relaxation times (DRT) to analyze the diffusion impedances of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C. The equivalent circuit diagram is shown in Supplementary information (Figure S6), where the Warburg impedance corresponds to the diffusion process of the material. From Figure 4D, we can find that the Warburg impedances of the three materials (MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG, and MnFe_PBA_Cryo_−10°C) decrease sequentially, indicating that the diffusion coefficient of MnFe_PBA_Cryo_−10°C is the highest, promoting ion diffusion. However, DRT analysis avoids the modeling of equivalent circuits and analyzes impedance in a model-free manner. In DRT, different peaks correspond to different reaction processes, and the height of the peaks corresponds to the difficulty of the reaction. As shown in Figure 4E, the peaks of log(τ) between −1 and −2 are the peaks of the diffusion process [57]. The peak heights of the diffusion processes of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG, and MnFe_PBA_Cryo_−10°C decrease successively, indicating that the diffusion coefficient of MnFe_PBA_Cryo_−10°C is the largest, which is conducive to the diffusion of ions. Meanwhile, in Figure 4E, the peak heights of the Rohm, RSEI, and Rct of MnFe_PBA_Cryo_−10°C are all lower than those of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG. In conclusion, materials with uniform particles are more favorable for ion diffusion.
 |
Figure 4 (A) Schematic diagram of electrolyte diffusion in PBS with non-uniform and uniform particles. (B) Simulation of the electrolyte diffusion in PBA with uniform and nonuniform particles. (C) GITT date for MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C with their respective diffusivities D. (D) The electrochemical impedance spectroscopy (EIS) of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C, and their corresponding Warburg impedances (inset). (E) The corresponding distribution of relaxation times (DRT) plot for MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG, and MnFe_PBA_Cryo_−10°C. |
Using the described cryo-synthesis method, the MnFe_PBA_Cryo_−10°C cathode exhibits excellent cycling stability and outstanding rate performance, prompting us to explore the practical applications. Previous studies have shown that organic 3,4,9,10-perylenetetracarboxylic diimide (PTCDI) has superior electrochemical performance in aqueous batteries. Therefore, we chose PTCDI as the anode and MnFe_PBA_Cryo_−10°C as the cathode for the full cell. In the rate performance graph of the MnFe_PBA_Cryo_−10°C || PTCDI full cell (Figure 5A and B), it can be seen that the full cell delivers reversible capacities of 88.2, 91, 87.5, 85.4, and 82.9 mAh g−1 at current densities of 500, 800, 1000, 1200, and 1500 mA g−1, respectively, indicating excellent rate capability. Significantly, when the current density decreases from 1500 to 500 mA g−1, the full cell can still provide a reversible 84.4 mAh g−1. As shown in Figure 5C, the MnFe_PBA_Cryo_−10°C || PTCDI full cell also exhibits an activation phenomenon at a current density of 500 mA g−1. The capacity retention is 80.3% and the average Coulombic efficiency approaches 100% over 1500 cycles. Furthermore, under room temperature conditions and at a current density of 200 mA g−1, the MnFe_PBA_Cryo_−10°C || PTCDI pouch cell demonstrates stable cycling performance with very little capacity decay in Figure 5D. To further validate the excellent performance of the MnFe_PBA_Cryo_−10°C material, we assembled a MnFe_PBA_Cryo_−10°C || PTCDI pouch cell and conducted constant current charge-discharge tests at various temperature conditions (Figure 5E). The results indicated that it could operate normally between −10°C and 30°C. In summary, the normal operation of the pouch cell suggests that the MnFe_PBA_Cryo_−10°C || PTCDI material has significant application potential in aqueous potassium-ion batteries.
 |
Figure 5 (A) Rate performance of the MnFe_PBA_Cryo_−10°C || PTCDI full battery at current densities in the range from 500 to 1500 mA g−1. (B) Charge-discharge curves of the MnFe_PBA_Cryo_−10°C || PTCDI full battery at current densities in the 500 to 1500 mA g−1 range. (C) Cycling performances of the MnFe_PBA_Cryo_−10°C || PTCDI full battery at a current density of 500 mA g−1. (D) Cycling performances of the MnFe_PBA_Cryo_−10°C || PTCDI pouch-type full battery at a current density of 200 mA g−1. (E) Performance of the MnFe_PBA_Cryo_−10°C || PTCDI pouch-type full battery at different temperatures (−10°C to 30°C) at the current density of 200 mA g−1. |
CONCLUSIONS
We have successfully developed a cryo-synthesis strategy for MnFe_PBA cathode material. By finely controlling the nucleation and growth processes through reduced synthesis temperatures, we synthesized MnFe_PBA_Cryo_−10°C with excellent electrochemical performance. This superior performance is attributed to the relatively uniform particle size distribution, excellent crystallinity fewer defects, and reduced water content in MnFe_PBA_Cryo_−10°C. Specifically, the MnFe_PBA_Cryo_−10°C exhibits a high capacity of 103 mAh g−1 (the average discharge capacity of MnFe_PBA_Cryo_−10°C after 3500 cycles at a current density of 500 mA g−1) and a capacity retention rate of 88% after 3500 cycles at a current density of 500 mA g−1. Furthermore, the MnFe_PBA_Cryo_−10°C cathode achieves remarkable long-term cycling stability of 10,000 cycles at a current density of 2000 mA g−1. Thus, MnFe_PBA_Cryo_−10°C with uniform particles has a higher diffusion coefficient, which is favorable for ion diffusion and thereby enhances the electrochemical performance of this cathode material. Interestingly, the MnFe_PBA_Cryo_−10°C || PTCDI pouch cell operated normally in the temperature range from −10°C to 30°C. Our research indicates that MnFe_PBA_Cryo_−10°C, synthesized via the cryo-synthesis method, offers competitive advantages among various cathode materials due to its long calendar life, low cost, and outstanding electrochemical properties, making it highly promising for practical applications.
Data availability
The original data are available from the corresponding authors upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (U20A20247, 51922038, 22302065 and 12104434), the National Key Research and Development Program of the Ministry of Science and Technology (2022YFA1402504), the Guangdong Basic and Applied Basic Research Foundation (2023A1515012176), the Science and Technology Innovation Program of Hunan Province (2024RC3081), and the Hunan Natural Science Foundation (2023JJ40140 and 2024JJ4009).
Author contributions
C.G. and B.L. conceived the concept. Z.Q. designed and carried out the experiments. W.L. and Y.L. performed the EIS and XPS analyses. A.M.R. conducted the analysis and discussion. Z.Q. and C.G. wrote the paper, and all authors discussed the results and commented on the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Supplementary file provided by the authors. Access here
References
- Wu L, Fu H, Li S, et al. Phase-engineered cathode for super-stable potassium storage. Nat Commun 2023; 14: 644. [Article] [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Wang H, Hu J, Dong J, et al. Artificial solid‐electrolyte interphase enabled high‐capacity and stable cycling potassium metal batteries. Adv Energy Mater 2019; 9: 1902697. [Article] [Google Scholar]
- Luo W, Feng Y, Shen D, et al. Engineering ion diffusion by CoS@SnS heterojunction for ultrahigh-rate and stable potassium batteries. ACS Appl Mater Interfaces 2022; 14: 16379-16385. [Article] [Google Scholar]
- Xia M, Feng Y, Wei J, et al. A rechargeable K/Br battery. Adv Funct Mater 2022; 32: 2205879. [Article] [Google Scholar]
- Dhir S, Wheeler S, Capone I, et al. Outlook on K-ion batteries. Chem 2020; 6: 2442-2460. [Article] [Google Scholar]
- Fan L, Xie H, Hu Y, et al. A tailored electrolyte for safe and durable potassium ion batteries. Energy Environ Sci 2023; 16: 305-315. [Article] [Google Scholar]
- Shen D, Rao AM, Zhou J, et al. High‐potential cathodes with nitrogen active centres for quasi‐solid proton‐ion batteries. Angew Chem Int Ed 2022; 61: e202201972. [Article] [Google Scholar]
- Li F, Luo W, Fu H, et al. Construct a quasi‐high‐entropy interphase for advanced low‐temperature aqueous zinc‐ion battery. Adv Funct Mater 2025; 35: 2416668. [Article] [Google Scholar]
- Zhang Z, Qiao Y, Zhao J, et al. Fluorine-doped K0.39Mn0.77Ni0.23O1.9F0.1 microspheres with highly reversible oxygen redox reaction for potassium-ion battery cathode. Chin Chem Lett 2025; 36: 109907. [Article] [Google Scholar]
- Gu ZY, Wang XT, Zhao XX, et al. Advanced K3V2(PO4)2O2F cathode for rechargeable potassium-ion batteries with high energy density. Appl Phys Lett 2024; 124: 183904. [Article] [Google Scholar]
- Zhang Q, Wang Z, Zhang S, et al. Cathode materials for potassium-ion batteries: Current status and perspective. Electrochem Energ Rev 2018; 1: 625-658. [Article] [Google Scholar]
- Wang H, Yu D, Wang X, et al. Electrolyte chemistry enables simultaneous stabilization of potassium metal and alloying anode for potassium‐ion batteries. Angew Chem Int Ed 2019; 58: 16451-16455. [Article] [Google Scholar]
- Hameed AS, Katogi A, Kubota K, et al. A layered inorganic-organic open framework material as a 4 V positive electrode with high‐rate performance for K‐ion batteries. Adv Energy Mater 2019; 9: 1902528. [Article] [Google Scholar]
- Lin H, Li M, Yang X, et al. Nanosheets‐assembled CuSe crystal pillar as a stable and high‐power anode for sodium‐ion and potassium‐ion batteries. Adv Energy Mater 2019; 9: 1900323. [Article] [Google Scholar]
- Heng, Gu, Liu, et al. Breaking anionic solvation barrier for safe and durable potassium‐ion batteries under ultrahigh‐voltage operation. Angew Chem Int Ed 2025; 64: e202423044. [Article] [Google Scholar]
- Liu Y, Gu ZY, Heng YL, et al. Interface defect induced upgrade of K-storage properties in KFeSO4F cathode: From lowered Fe-3d orbital energy level to advanced potassium-ion batteries. Green Energy Environ 2024; 9: 1724-1733. [Article] [Google Scholar]
- Xiao Z, Meng J, Xia F, et al. K+ modulated K+/vacancy disordered layered oxide for high-rate and high-capacity potassium-ion batteries. Energy Environ Sci 2020; 13: 3129-3137. [Article] [Google Scholar]
- Huang J, Cai X, Yin H, et al. A new candidate in polyanionic compounds for a potassium-ion battery cathode: KTiOPO4. J Phys Chem Lett 2021; 12: 2721-2726. [Article] [Google Scholar]
- Kim H, Ji H, Wang J, et al. Next-generation cathode materials for non-aqueous potassium-ion batteries. Trends Chem 2019; 1: 682-692. [Article] [Google Scholar]
- Li L, Hu Z, Lu Y, et al. A low‐strain potassium‐rich prussian blue analogue cathode for high power potassium‐ion batteries. Angew Chem 2021; 133: 13160-13166. [Article] [Google Scholar]
- Xie B, Sun B, Gao T, et al. Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries. Coord Chem Rev 2022; 460: 214478. [Article] [Google Scholar]
- Yang Y, Zhou J, Wang L, et al. Prussian blue and its analogues as cathode materials for Na-, K-, Mg-, Ca-, Zn- and Al-ion batteries. Nano Energy 2022; 99: 107424. [Article] [Google Scholar]
- Shu W, Han C, Wang X. Prussian blue analogues cathodes for nonaqueous potassium‐ion batteries: Past, present, and future. Adv Funct Mater 2024; 34: 2309636. [Article] [Google Scholar]
- Zakaria MB, Chikyow T. Recent advances in Prussian blue and Prussian blue analogues: Synthesis and thermal treatments. Coord Chem Rev 2017; 352: 328-345. [Article] [Google Scholar]
- Gao Y, Wu X, Wang L, et al. Structurally stable, low H2O Prussian blue analogs toward high performance sodium storage. Adv Funct Mater 2024; 34: 2314860. [Article] [Google Scholar]
- Peng J, Zhang W, Hu Z, et al. Ice-assisted synthesis of highly crystallized prussian blue analogues for all-climate and long-calendar-life sodium ion batteries. Nano Lett 2022; 22: 1302-1310. [Article] [Google Scholar]
- Deng L, Qu J, Niu X, et al. Defect-free potassium manganese hexacyanoferrate cathode material for high-performance potassium-ion batteries. Nat Commun 2021; 12: 2167. [Article] [Google Scholar]
- Zhou A, Xu Z, Gao H, et al. Size‐, water‐, and defect‐regulated potassium manganese hexacyanoferrate with superior cycling stability and rate capability for low‐cost sodium‐ion batteries. Small 2019; 15: 1902420. [Article] [Google Scholar]
- Liu Y, Chu W, Xu Y, et al. The effect of coprecipitation and heating temperature on structural evolution and electrochemical performance of iron-based prussian blue analogs. J Solid State Electrochem 2024; 28: 4105-4118. [Article] [Google Scholar]
- Kim J, Yi, Li L, et al. Effect of valence state of cobalt in cobalt hexacyanoferrate coprecipitated at different temperatures on electrochemical behavior. Intl J Energy Res 2022; 46: 22717-22729. [Article] [Google Scholar]
- Erdemir D, Lee AY, Myerson AS. Nucleation of crystals from solution: Classical and two-step models. Acc Chem Res 2009; 42: 621-629. [Article] [Google Scholar]
- Li J, Deepak FL. In situ kinetic observations on crystal nucleation and growth. Chem Rev 2022; 122: 16911-16982. [Article] [Google Scholar]
- Zhang JB, Zhang PY, Ma K, et al. Hydrogen bonding interactions between ethylene glycol and water: Density, excess molar volume, and spectral study. Sci China Ser B-Chem 2008; 51: 420-426. [Article] [Google Scholar]
- Yang DH, Yao ZQ, Wu D, et al. Structure-modulated crystalline covalent organic frameworks as high-rate cathodes for Li-ion batteries. J Mater Chem A 2016; 4: 18621-18627. [Article] [Google Scholar]
- Gao C, Liu Y, Zheng L, et al. The effect of electrolyte type on the Li ion intercalation in copper hexacyanoferrate. J Electrochem Soc 2019; 166: A1732-A1737. [Article] [Google Scholar]
- Gerber SJ, Erasmus E. Electronic effects of metal hexacyanoferrates: An XPS and FTIR study. Mater Chem Phys 2018; 203: 73-81. [Article] [Google Scholar]
- Xiang S, Zhang X, Tao Q, et al. Adsorption of cesium on mesoporous SBA-15 material containing embedded copper hexacyanoferrate. J Radioanal Nucl Chem 2019; 320: 609-619. [Article] [Google Scholar]
- Ma XH, Jia W, Wang J, et al. Synthesis of copper hexacyanoferrate nanoflake as a cathode for sodium-ion batteries. Ceram Int 2019; 45: 740-746. [Article] [Google Scholar]
- Xia M, Zhang X, Liu T, et al. Commercially available Prussian blue get energetic in aqueous K-ion batteries. Chem Eng J 2020; 394: 124923. [Article] [Google Scholar]
- Su D, McDonagh A, Qiao, et al. High‐capacity aqueous potassium‐ion batteries for large‐scale energy storage. Adv Mater 2017; 29: 1604007. [Article] [Google Scholar]
- Zhang L, Chen L, Zhou X, et al. Towards high‐voltage aqueous metal‐ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system. Adv Energy Mater 2015; 5: 1400930. [Article] [Google Scholar]
- Ren W, Chen X, Zhao C. Ultrafast aqueous potassium‐ion batteries cathode for stable intermittent grid‐scale energy storage. Adv Energy Mater 2018; 8: 1801413. [Article] [Google Scholar]
- Zhu K, Li Z, Jin T, et al. Low defects potassium cobalt hexacyanoferrate as a superior cathode for aqueous potassium ion batteries. J Mater Chem A 2020; 8: 21103-21109. [Article] [Google Scholar]
- Han J, Mariani A, Zhang H, et al. Gelified acetate-based water-in-salt electrolyte stabilizing hexacyanoferrate cathode for aqueous potassium-ion batteries. Energy Storage Mater 2020; 30: 196-205. [Article] [Google Scholar]
- Shang Y, Li X, Song J, et al. Unconventional Mn vacancies in Mn-Fe prussian blue analogs: Suppressing Jahn-Teller distortion for ultrastable sodium storage. Chem 2020; 6: 1804-1818. [Article] [Google Scholar]
- Chen X, Hua C, Zhang K, et al. Control of gradient concentration Prussian white cathodes for high-performance potassium-ion batteries. ACS Appl Mater Interfaces 2023; 15: 47125-47134. [Article] [Google Scholar]
- Wu X, Sun M, Guo S, et al. Vacancy‐free prussian blue nanocrystals with high capacity and superior cyclability for aqueous sodium‐ion batteries. ChemNanoMat 2015; 1: 188-193. [Article] [Google Scholar]
- Wang Z, Zhuo W, Li J, et al. Regulation of ferric iron vacancy for Prussian blue analogue cathode to realize high-performance potassium ion storage. Nano Energy 2022; 98: 107243. [Article] [Google Scholar]
- Wang W, Gang Y, Hu Z, et al. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat Commun 2020; 11: 980. [Article] [Google Scholar]
- Wu X, Cao Y, Ai X, et al. A low-cost and environmentally benign aqueous rechargeable sodium-ion battery based on NaTi2(PO4)3-Na2NiFe(CN)6 intercalation chemistry. Electrochem Commun 2013; 31: 145-148. [Article] [Google Scholar]
- Li W, Zhang F, Xiang X, et al. Nickel‐substituted copper hexacyanoferrate as a superior cathode for aqueous sodium‐ion batteries. ChemElectroChem 2018; 5: 350-354. [Article] [Google Scholar]
- Wang B, Wang X, Liang C, et al. An all‐prussian‐blue‐based aqueous sodium‐ion battery. ChemElectroChem 2019; 6: 4848-4853. [Article] [Google Scholar]
- Trócoli R, La Mantia F. An aqueous zinc‐ion battery based on copper hexacyanoferrate. ChemSusChem 2015; 8: 481-485. [Article] [Google Scholar]
- Bi H, Wang X, Liu H, et al. A universal approach to aqueous energy storage via ultralow‐cost electrolyte with super‐concentrated sugar as hydrogen‐bond‐regulated solute. Adv Mater 2020; 32: 2000074. [Article] [Google Scholar]
- Lu K, Zhang H, Gao S, et al. High rate and stable symmetric potassium ion batteries fabricated with flexible electrodes and solid-state electrolytes. Nanoscale 2018; 10: 20754-20760. [Article] [Google Scholar]
- Wessells CD, McDowell MT, Peddada SV, et al. Tunable reaction potentials in open framework nanoparticle battery electrodes for grid-scale energy storage. ACS Nano 2012; 6: 1688-1694. [Article] [Google Scholar]
- Lu Y, Zhao CZ, Huang JQ, et al. The timescale identification decoupling complicated kinetic processes in lithium batteries. Joule 2022; 6: 1172-1198. [Article] [Google Scholar]
All Figures
 |
Figure 1 Schematic illustration of nucleation-growth mechanisms in Prussian blue analogues (PBAs) synthesis and their temperature-dependent crystallization control. (A) PBA’s nucleation and growth kinetics are largely governed by temperature, where Tf and Tm1/Tm2 represent the solidification temperature (addition EG) and the fastest rate of nucleation/growth temperature of the precursor solution. (B) The scenarios of PBA synthesis under different temperatures. Conventional synthesis routes exhibit limited control over particle size distribution due to rapid nucleation under ambient conditions. Thermodynamically favored growth leads to particle aggregation with heterogeneous size distribution at room temperature (upper panel). The sub-zero synthesis cannot be achieved due to the freezing of the aqueous precursor solution (middle panel). Our developed cryo-synthesis approach introduces hydrogen-bond disrupting additives, achieving monodisperse nanocrystals with enhanced crystallinity (lower panel). |
| In the text | |
 |
Figure 2 Structural and compositional characterization of MnFe_PBA synthesized under ambient and cryogenic conditions. (A–F) The XRD patterns and the Rietveld refinement of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C. The SEM images and the statistics of the particle size distribution of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C, respectively. (G) Transmission electron microscopy (TEM) image of MnFe_PBA_cryo_−10°C. The XPS fine scan of Mn 2p (H) and Fe 2p (I) spectra of MnFe_PBA_cryo_−10°C. |
| In the text | |
 |
Figure 3 The electrochemical performances of MnFe_PBA synthesized at ambient and −10°C. (A) Cyclic voltammetry (CV) curves of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG, and MnFe_PBA_Cryo_−10°C with their respective polarization voltages. (B) Cycling performances of MnFe_PBA at room temperature and cryo-synthesis at the current densities of 500 mA g−1. (C) Rate performance of MnFe_PBA_Cryo_−10°C at current densities from 500 to 1500 mA g−1. (D) Charge-discharge curves of MnFe_PBA_Cryo_−10°C at current densities from 500 to 1500 mA g−1. (E) Long-term cycling performances of MnFe_PBA_Cryo_−10°C at the current densities of 2000 mA g−1. (F) Comparison of the capacity retention in this work with previously reported PBAs. |
| In the text | |
 |
Figure 4 (A) Schematic diagram of electrolyte diffusion in PBS with non-uniform and uniform particles. (B) Simulation of the electrolyte diffusion in PBA with uniform and nonuniform particles. (C) GITT date for MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C with their respective diffusivities D. (D) The electrochemical impedance spectroscopy (EIS) of MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG and MnFe_PBA_Cryo_−10°C, and their corresponding Warburg impedances (inset). (E) The corresponding distribution of relaxation times (DRT) plot for MnFe_PBA_RT w/o EG, MnFe_PBA_RT w EG, and MnFe_PBA_Cryo_−10°C. |
| In the text | |
 |
Figure 5 (A) Rate performance of the MnFe_PBA_Cryo_−10°C || PTCDI full battery at current densities in the range from 500 to 1500 mA g−1. (B) Charge-discharge curves of the MnFe_PBA_Cryo_−10°C || PTCDI full battery at current densities in the 500 to 1500 mA g−1 range. (C) Cycling performances of the MnFe_PBA_Cryo_−10°C || PTCDI full battery at a current density of 500 mA g−1. (D) Cycling performances of the MnFe_PBA_Cryo_−10°C || PTCDI pouch-type full battery at a current density of 200 mA g−1. (E) Performance of the MnFe_PBA_Cryo_−10°C || PTCDI pouch-type full battery at different temperatures (−10°C to 30°C) at the current density of 200 mA g−1. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

